Paracetamol: Introduction
Paracetamol, also known as Panadol, Calpol, or Alvedon, is an analgesic and antipyretic medication that is used to temporarily alleviate mild-to-moderate pain and fever. It is commonly included as an ingredient in cold and flu medications and can also be used individually. Paracetamol and acetaminophen (Tylenol) are the same drugs but they are named differently in different countries. Paracetamol is the name assigned using the International Nonproprietary Name (INN) system, while acetaminophen is the name assigned using the United States Adopted Names (USAN) system. Paracetamol is used in Europe, Australia, New Zealand, and India, while acetaminophen is used in countries such as the United States, Canada, and Japan. Generally, the INN and USAN names for a drug are the same and do not differ between countries.Global Paracetamol Market Analysis
The global market for paracetamol has experienced significant growth and is poised for steady growth in the coming years as well. The market is poised to grow due to recent developments for example, Dr. Bhaumik Pandya's innovative formulation, aimed at combating liver toxicity associated with paracetamol (PCM), is a significant innovation. By increasing PCM's water solubility, this patented innovation not only enhances safety by preventing liver damage but also enables precise dosing. This will enable patients to take the exact required dosage, such as 250mg, instead of opting for higher doses like 500mg or 650mg, potentially revolutionizing the consumption pattern of paracetamol. This advancement could lead to increased demand for this novel formulation, influencing the global paracetamol market growth.In August 2023, the market for paracetamol faced a challenge when the prices of its key ingredients suddenly surged. This caught both pharmaceutical companies and healthcare professionals off guard. The unexpected cost escalation could hinder the market growth, leading to either higher retail prices or supply chain disruptions. To mitigate these effects, pharmaceutical companies may need to increase their research and development efforts to create more cost-effective versions of paracetamol. This could stimulate innovation but might also introduce market volatility. Overall, while Dr. Pandya's invention offers a promising way to expand the market and improve patient safety, the increase in ingredient prices presents a hurdle that the industry must overcome to sustain and promise market growth.
Global Paracetamol Market Segmentations
“global paracetamol market and forecast 2025-2034” offers a detailed analysis of the market based on the following segments:Market Breakup by Dosage Type
- Tablet
- Capsules
- Others
Market Breakup by Applications
- Headache and Fever
- Cold and Cough
- Muscle Cramps
- Others
Market Breakup by Region
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East and Africa
Global Paracetamol Market Overview
The market has been witnessing significant market growth due to the rising frequency of seasonal flu causing cold and fever coupled with body pain. Paracetamol is used as a primary treatment for mild to moderate cases of fever, headaches, and body pain among others. The rise in frequent cases of fever and cold among the geriatric population, adults, and pediatrics is leading to increased paracetamol market demand.The convenience of over the counter (OTC) availability of the medication contributes to the increased demand, particularly for common pain and fever relief, adding further value to the paracetamol market share. Paracetamol is often combined with other active ingredients to address multiple symptoms in a single medication (e.g., paracetamol with decongestants for cold relief). Due to the requirement of paracetamol in such combinational dosages, the market is expected to keep expanding for the forecast period.
Global Paracetamol Market: Competitor Landscape
The key features of the market report include patent analysis, grants analysis, clinical trials analysis, funding and investment analysis, partnerships, and collaborations analysis by the leading key players. The major companies in the market are as follows:- GlaxoSmithKline plc
- Teva Pharmaceuticals USA, Inc.
- Sanofi
- Mallinckrodt Pharmaceuticals
- Sun Pharmaceutical Industries Ltd.
- Geno Pharma
- Biological E
- CFL Pharma
- Cipla
- Dr. Reddy's Lab
This product will be delivered within 3-5 business days.
Table of Contents
Companies Mentioned
- GlaxoSmithKline plc
- Teva Pharmaceuticals USA, Inc.
- Sanofi
- Mallinckrodt Pharmaceuticals
- Sun Pharmaceutical Industries Ltd.
- Geno Pharma
- Biological E
- CFL Pharma
- Cipla
- Dr. Reddy’s Lab
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 350 |
| Published | July 2025 |
| Forecast Period | 2025 - 2034 |
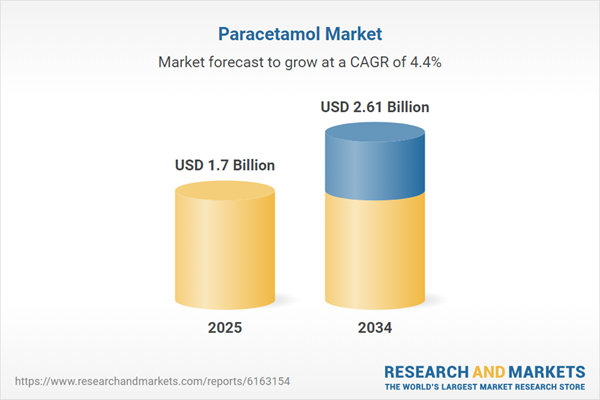
| Estimated Market Value ( USD | $ 1.7 Billion |
| Forecasted Market Value ( USD | $ 2.61 Billion |
| Compound Annual Growth Rate | 4.4% |
| Regions Covered | Global |
| No. of Companies Mentioned | 10 |