Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
Despite these positive factors, market expansion faces a major obstacle in the form of the high cost of long-term antifibrotic treatments, which hinders patient access in healthcare systems with strict reimbursement policies. This financial hurdle, often compounded by stringent payer approval criteria, restricts the broad utilization of these life-extending therapies across both developed and emerging regions.
Market Drivers
The rising prevalence of Idiopathic Pulmonary Fibrosis (IPF) acts as a key determinant of market progress, requiring a corresponding increase in therapeutic availability and patient support infrastructures. Improvements in diagnostic capabilities enable healthcare providers to detect more instances of this chronic, progressive lung condition, thereby escalating the demand for long-term management strategies. This increasing disease burden is a global health issue rather than a regional one, fueling the commercial need for effective treatments; for instance, the Canadian Pulmonary Fibrosis Foundation’s 'Hope Breathes Here Press Release' in September 2024 noted that approximately 30,000 Canadians are currently living with pulmonary fibrosis, underscoring the large patient population needing care.Concurrently, the rapid uptake of advanced antifibrotic therapies significantly boosts market value, as these medications remain the standard of care for retarding disease progression. The commercial viability of existing agents stimulates ongoing research and development, fostering a competitive landscape focused on efficacy and safety. The strong demand for pharmaceutical interventions is reflected in financial results; Boehringer Ingelheim’s April 2024 'Annual Report 2023' reported net sales of 3.5 billion EUR for their fibrosis portfolio, led by Ofev. Furthermore, the market continues to attract investment for future innovations, as demonstrated by Vicore Pharma, which raised approximately 782 million SEK in a 2024 rights issue to progress its lead candidate through clinical trials.
Market Challenges
The exorbitant cost associated with long-term antifibrotic therapy represents a major economic hurdle slowing the growth of the Global Idiopathic Pulmonary Fibrosis (IPF) Market. Although these medications effectively retard disease progression, their high prices often surpass the financial capacity of both patients and healthcare systems. As a result, insurance providers and payers frequently enforce rigorous reimbursement standards, such as necessitating proof of lung function decline prior to approval, which delays the start of treatment and shrinks the eligible patient pool, ultimately lowering revenue potential.This financial toxicity not only hinders new patient enrollment but also leads to high rates of treatment abandonment. Patients confronted with significant out-of-pocket costs are often compelled to prioritize essential living expenses over medication adherence, resulting in poorer clinical outcomes and decreased drug sales volume. Highlighting this burden, the Pulmonary Fibrosis Foundation noted in 2024 that Medicare beneficiaries in the United States faced an annual out-of-pocket spending cap of roughly $8,000 for prescription drugs before new limits were enacted, demonstrating the severe economic strain on the elderly population most susceptible to the disease. These economic pressures create a restrictive atmosphere that suppresses the widespread uptake of therapeutics, thereby impeding overall market expansion.
Market Trends
The integration of Artificial Intelligence into diagnostic imaging is transforming the Global Idiopathic Pulmonary Fibrosis Market by substituting subjective manual evaluations with automated, quantitative biomarkers that more accurately predict disease progression. These AI-powered tools examine computed tomography scans to measure variations in lung structure, equipping clinicians with reliable data for patient stratification in clinical trials and personalized care. According to a March 2025 report by the National Institutes of Health on highlights from the 'ERS Congress 2024', the AI-derived Weighted Reticulovascular Score (WRVS) showed superior prognostic ability over traditional densitometry, identifying patients with a high mortality risk through a hazard ratio of 4.55.Additionally, the rise of Next-Generation Mechanism-Specific Therapies marks a critical transition from broad-spectrum antifibrotics to precision agents that target specific pathogenic pathways, such as lysophosphatidic acid signaling. This trend is motivated by the critical need to enhance patient adherence and results via drugs that provide improved safety profiles and targeted efficacy compared to legacy treatments like pirfenidone and nintedanib. As reported by Chest Physician in July 2025 regarding the article 'Admilparant phase 2 results raise hopes for combination treatment in pulmonary fibrosis', the investigational LPA1 antagonist admilparant resulted in a 54% relative risk reduction in lung function decline among idiopathic pulmonary fibrosis patients treated with the 60 mg dose.
Key Players Profiled in the Idiopathic Pulmonary Fibrosis Market
- Boehringer Ingelheim International GmbH
- F. Hoffmann-La Roche Ltd.
- Cipla Limited
- Shionogi & Co., Ltd.
- Bristol-Myers Squibb Company
- United Therapeutics Corporation
- FibroGen, Inc.
- Pliant Therapeutics, Inc.
- Galecto, Inc.
- CSL Behring
Report Scope
In this report, the Global Idiopathic Pulmonary Fibrosis Market has been segmented into the following categories:Idiopathic Pulmonary Fibrosis Market, by Drug Type:
- Pirfenidone
- Nintedanib
- others
Idiopathic Pulmonary Fibrosis Market, by Route of Administration:
- Parenteral
- Oral
- Others
Idiopathic Pulmonary Fibrosis Market, by Distribution Channel:
- Hospital Pharmacies
- Retail Pharmacies
- Online Pharmacies
Idiopathic Pulmonary Fibrosis Market, by Region:
- North America
- Europe
- Asia-Pacific
- South America
- Middle East & Africa
Competitive Landscape
Company Profiles: Detailed analysis of the major companies present in the Global Idiopathic Pulmonary Fibrosis Market.Available Customization
The analyst offers customization according to your specific needs. The following customization options are available for the report:- Detailed analysis and profiling of additional market players (up to five).
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
The key players profiled in this Idiopathic Pulmonary Fibrosis market report include:- Boehringer Ingelheim International GmbH
- F. Hoffmann-La Roche Ltd.
- Cipla Limited
- Shionogi & Co., Ltd
- Bristol-Myers Squibb Company
- United Therapeutics Corporation
- FibroGen, Inc
- Pliant Therapeutics, Inc
- Galecto, Inc.
- CSL Behring
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 185 |
| Published | January 2026 |
| Forecast Period | 2025 - 2031 |
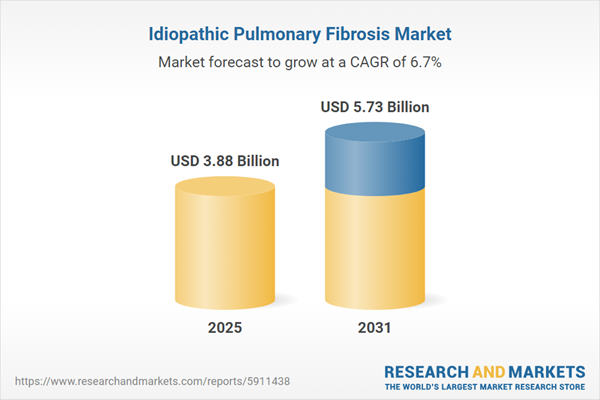
| Estimated Market Value ( USD | $ 3.88 Billion |
| Forecasted Market Value ( USD | $ 5.73 Billion |
| Compound Annual Growth Rate | 6.7% |
| Regions Covered | Global |
| No. of Companies Mentioned | 11 |