Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
Omics encompasses a range of high-throughput techniques used in biology and medicine to analyze biological molecules at the molecular level. These techniques include genomics (the study of genes and DNA), proteomics (the study of proteins), metabolomics (the study of small molecules and metabolites), transcriptomics (the study of RNA and gene expression), and more. A key objective of omics-based clinical trials is to stratify patients into subgroups based on their molecular profiles, enabling more targeted and personalized healthcare interventions.
However, omics-based clinical trials face challenges such as data standardization, reproducibility issues, and the need for advanced bioinformatics capabilities. The accuracy and reliability of omics data are crucial for successful trials. For example, an article published in April 2024 highlights the growing significance of omics-based biomarkers in managing metabolic dysfunction-associated steatotic liver disease (MASLD), where these biomarkers aid in risk stratification and identifying patients with advanced fibrosis who are at higher risk of adverse outcomes. The increasing adoption of omics-based clinical trials is expected to further propel market growth.
Key Market Drivers:
Advancements in Omics Technologies
Recent developments in high-throughput sequencing have made these techniques faster, more precise, and cost-effective. Technologies like single-cell sequencing enable the examination of genetic material at an individual cell level, revealing cellular heterogeneity. Mass spectrometry tools have become more sensitive and precise, allowing the identification and quantification of proteins and metabolites in complex biological samples.Additionally, advancements in mass spectrometry imaging (MSI) enable the spatial mapping of molecules within tissues. Cryo-electron microscopy (cryo-EM) has revolutionized the structural analysis of proteins, achieving near-atomic resolution. X-ray crystallography and nuclear magnetic resonance (NMR) spectroscopy continue to enhance molecular structure resolution. Technologies such as stable isotope labeling and flux analysis offer insights into metabolic pathways, while quantitative proteomics methods, including isobaric labeling and label-free approaches, allow precise protein quantification.
A report from the Mayo Clinic in February 2023 highlights a new approach in genomics research, shifting some omics studies from traditional settings like hospitals to individuals' homes, particularly in rural and underserved areas. This change is expected to improve access to clinical trials, expanding participant pools and enabling more diverse data collection.
Key Market Challenges:
Sample Size and Diversity
One of the challenges of omics-based clinical trials is obtaining an adequate number of patient samples, particularly for rare diseases or genetically specific subpopulations. The availability of diverse patient samples is crucial, as these trials often require large and varied cohorts to generate reliable results. Biological variability among patients within the same disease category can be substantial, making it necessary to include larger sample sizes for statistical validity.Furthermore, ensuring genetic and ethnic diversity in clinical trial populations is essential for the broader applicability of findings. Omics-based trials often involve subgroup analysis based on genetic or molecular characteristics, and recruiting patients that meet these specific criteria can be time-consuming and resource-intensive. Delays in recruitment and data collection can hinder the trial process. Additionally, obtaining informed consent for genetic and molecular profiling, as well as addressing privacy concerns, can also present challenges.
The integration and interpretation of large, complex omics data sets also require advanced bioinformatics tools, which can further complicate the analysis, especially when dealing with large and diverse sample sizes.
Key Market Trends:
Integration of Multi-Omics Data
Different omics technologies provide unique perspectives on the molecular mechanisms underlying diseases. Integrating data from multiple omics disciplines offers a more comprehensive view of disease processes by examining the interplay of genes, proteins, metabolites, and other factors. This integration enhances the identification and validation of biomarkers for disease diagnosis, prognosis, and treatment response. Combining genetic, proteomic, and metabolic profiles leads to more accurate and robust biomarker discovery.The integration of multi-omics data is pivotal for patient stratification, allowing researchers to classify patients based on molecular profiles and design more targeted clinical trials. This approach also facilitates the development of personalized therapies tailored to individual genetic, proteomic, and metabolic characteristics, leading to more effective treatments.
In drug development, multi-omics integration helps to understand how drugs interact with various molecular components, providing insights into potential adverse effects and guiding drug design. For complex diseases like cancer, multi-omics data integration aids in understanding intricate molecular interactions, facilitating the identification of novel therapeutic targets and strategies. This integration aligns with a systems biology approach, offering a holistic understanding of biological systems as interconnected networks of genes, proteins, and metabolites.
Key Market Players:
- Parexel International Corporation
- Pharmaceutical Product Development (PPD)
- Charles River Laboratory
- ICON plc
- SGS SA
- Eli Lilly and Company
- Pfizer Inc.
- Covance Inc.
- Novo Nordisk
- Rebus Bio
Report Scope:
This report segments the global omics-based clinical trials market into various categories and provides a comprehensive analysis of market trends:
By Phase:
- Phase I
- Phase II
- Phase III
- Phase IV
By Study Design:
- Expanded Access Studies
- Interventional Studies
- Observational Studies
By Indication:
- Cardiology
- CNS Diseases
- Genetic Diseases
- Immunology
- Oncology
- Respiratory Diseases
- Skin Diseases
By Region:
North America:
United States, Canada, MexicoAsia-Pacific:
China, India, South Korea, Australia, JapanEurope:
Germany, France, United Kingdom, Spain, ItalySouth America:
Brazil, Argentina, ColombiaMiddle East & Africa:
South Africa, Saudi Arabia, UAECompetitive Landscape:
This section provides detailed analysis and profiles of the major players in the global omics-based clinical trials market.Available Customizations:
TechSci Research offers tailored customization options for this market report. These include a detailed analysis of additional market players (up to five) as per specific company needs.This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
- Parexel International Corporation
- Pharmaceutical Product Development (PPD)
- Charles River Laboratory
- ICON plc
- SGS SA
- Eli Lilly and Company
- Pfizer Inc.
- Covance Inc.
- Novo Nordisk
- Rebus Bio
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 180 |
| Published | March 2025 |
| Forecast Period | 2024 - 2030 |
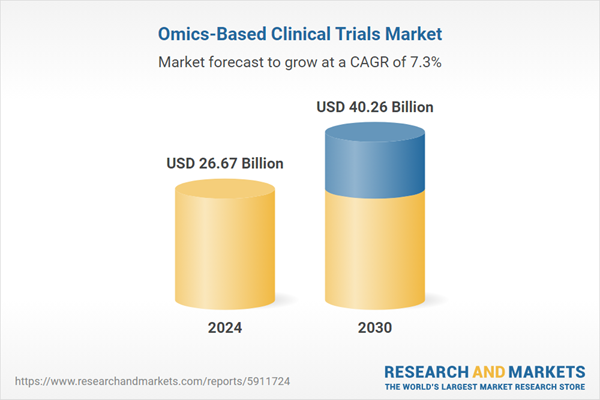
| Estimated Market Value ( USD | $ 26.67 Billion |
| Forecasted Market Value ( USD | $ 40.26 Billion |
| Compound Annual Growth Rate | 7.2% |
| Regions Covered | Global |
| No. of Companies Mentioned | 10 |