Europe In-Vitro Diagnostics Market Analysis
The Europe in-vitro diagnostics (IVD) market is a crucial segment of the broader medical diagnostics industry, providing vital tools for the detection and management of diseases. In-vitro diagnostics involve tests performed on samples such as blood, urine, or tissue that are taken from the human body. These tests are essential for disease diagnosis, treatment monitoring, and overall health management. The European market for IVD is known for its advanced technology and high adoption rate of innovative diagnostic solutions, driven by a well-established healthcare infrastructure and increasing awareness about early disease detection.Market Drivers
Technological Advancements: The continuous innovation in diagnostic technologies, including molecular diagnostics, next-generation sequencing (NGS), and point-of-care (POC) testing, has significantly enhanced the accuracy and speed of diagnostics. These advancements have facilitated early disease detection and personalised treatment plans.Ageing Population: Europe has a significant ageing population, leading to a higher prevalence of chronic diseases such as diabetes, cardiovascular diseases, and cancer. The growing need for efficient and accurate diagnostics to manage these conditions is a major driver for the IVD market.
Government Initiatives and Funding: European governments and health organisations are actively promoting the adoption of advanced diagnostics through funding and supportive regulations. This has encouraged research and development activities and the integration of new technologies into the healthcare system.
Rise in Infectious Diseases: The recent outbreaks of infectious diseases, including COVID-19, have highlighted the importance of robust diagnostic systems. The pandemic has accelerated the demand for rapid and accurate diagnostic tests, thereby boosting the IVD market.
Challenges
Regulatory Hurdles: The stringent regulatory framework in Europe can delay the approval and market entry of new diagnostic tests. Compliance with diverse regulations across different countries adds to the complexity and cost for manufacturers.High Costs: The development and implementation of advanced diagnostic technologies can be expensive. High costs associated with these technologies may limit their adoption, especially in lower-income regions within Europe.
Data Privacy Concerns: With the increasing use of digital platforms and electronic health records, data privacy and security have become significant concerns. Ensuring compliance with the General Data Protection Regulation (GDPR) and protecting patient information is a critical challenge.
Market Fragmentation: The European IVD market is highly fragmented, with numerous small and medium-sized enterprises (SMEs) alongside large multinational corporations. This fragmentation can lead to intense competition and pricing pressures.
Future Opportunities
Personalised Medicine: The shift towards personalised medicine, which tailors treatment based on individual patient characteristics, presents significant growth opportunities. Advanced IVD tests that provide detailed genetic and molecular information are crucial for personalised treatment plans.Integration of AI and Machine Learning: The integration of artificial intelligence (AI) and machine learning (ML) into diagnostic tools can enhance the accuracy and efficiency of diagnostics. These technologies can assist in data analysis, predictive diagnostics, and decision-making processes.
Expansion of Point-of-Care Testing: Point-of-care testing (POCT) offers immediate results and can be conducted at or near the site of patient care. The expansion of POCT in primary care settings, remote areas, and home healthcare can significantly improve access to diagnostics.
Collaborations and Partnerships: Collaborations between diagnostic companies, research institutions, and healthcare providers can drive innovation and accelerate the development of new diagnostic solutions. Strategic partnerships can also help in expanding market reach and enhancing product offerings.
Emerging Markets: While Western Europe dominates the IVD market, there are substantial growth opportunities in Eastern Europe. Improving healthcare infrastructure and increasing healthcare expenditure in these regions can drive the adoption of advanced diagnostic technologies.
Europe In-Vitro Diagnostics Market Trends
The Europe in-vitro diagnostics (IVD) market is evolving rapidly, influenced by technological innovations and changing healthcare dynamics. Here are some insightful market trends shaping the future of this industry:Market Trends
Rise of Molecular Diagnostics: Molecular diagnostics are becoming increasingly important due to their precision and ability to detect genetic mutations and infectious agents at an early stage. Techniques such as polymerase chain reaction (PCR) and next-generation sequencing (NGS) are widely adopted, offering rapid and accurate diagnostics, which are crucial for personalised medicine and targeted therapies.Growth in Point-of-Care Testing (POCT): The demand for point-of-care testing is on the rise, driven by the need for immediate diagnostic results, especially in critical care and emergency settings. POCT enables faster decision-making and treatment initiation, improving patient outcomes. The trend towards decentralised healthcare and home testing also supports the growth of POCT.
Integration of Artificial Intelligence (AI) and Machine Learning (ML): AI and ML are revolutionising the IVD market by enhancing the accuracy and efficiency of diagnostic processes. These technologies are used for data analysis, pattern recognition, and predictive diagnostics, aiding in early disease detection and personalised treatment plans. AI-powered diagnostic tools are becoming more prevalent in clinical laboratories and hospitals.
Adoption of Digital Pathology: Digital pathology is transforming traditional pathology by enabling the digitisation of slides and data for analysis. This trend allows pathologists to analyse samples remotely, collaborate more effectively, and utilise AI tools for better diagnostic accuracy. Digital pathology enhances workflow efficiency and is gaining traction in Europe.
Personalised Medicine and Companion Diagnostics: The move towards personalised medicine is driving the development of companion diagnostics, which are tests designed to determine the suitability of specific treatments for individual patients. This trend is particularly significant in oncology, where targeted therapies require precise diagnostics to identify suitable candidates for treatment.
Europe In-Vitro Diagnostics Market Segmentation
Market Breakup by Product Type
- Reagents and Kits
- Instruments
- Software and Services
Market Breakup by Technology
- Immunoassay/ Immunochemistry
- Clinical Chemistry
- Molecular Diagnostics
- Haematology
- Microbiology
- Blood Glucose Self-Monitoring
- Coagulation and Haemostasis
- Urinalysis
- Others
Market Breakup by Therapeutic Area
- Infectious Diseases
- Diabetes
- Cardiology
- Oncology
- Autoimmune Diseases
- Nephrology
- Others
Market Breakup by End User
- Hospitals
- Laboratories
- Homecare
- Others
Europe In-Vitro Diagnostics Market Competitive Landscape
The Europe in-vitro diagnostics (IVD) market is highly competitive, with key players including F. Hoffmann-La Roche Ltd, QIAGEN GmbH, BioMérieux, Inc., Siemens Healthineers AG, ELITechGroup, Oncgnostics GmbH, Abbott Laboratories, Danaher Corporation, Sysmex Corporation, and Thermo Fisher Scientific Inc. These companies engage in common market activities such as mergers and acquisitions to expand their market presence and capabilities. They are also heavily involved in research initiatives to innovate and improve diagnostic technologies. Frequent product introductions and partnerships with other firms and research institutions are strategies used to enhance their offerings and market reach, driving competitive growth and technological advancement in the European IVD market.Key Questions Answered in the Report
- What is the current and future performance of the Europe In-Vitro Diagnostics market?
- What are the main challenges facing the Europe In-Vitro Diagnostics market?
- What are the key drivers of the Europe In-Vitro Diagnostics market?
- What emerging trends are shaping the future of the Europe In-Vitro Diagnostics market?
- How is the trend towards personalised medicine influencing the development of companion diagnostics?
- Why do reagents and kits dominate the IVD market, and how are instruments evolving?
- How are molecular diagnostics, clinical chemistry, and haematology evolving in the IVD market?
- What factors are driving the sustained demand for diabetes diagnostics?
- Why do hospitals dominate the IVD market in terms of diagnostic test volumes?
Key Benefits for Stakeholders
- The industry report offers a comprehensive quantitative analysis of various market segments, historical and current market trends, market forecasts, and dynamics of the Europe In-Vitro Diagnostics market from 2017-2032.
- The research report provides the latest information on the market drivers, challenges, and opportunities in the Europe In-Vitro Diagnostics market.
- The study maps the leading, as well as the fastest-growing, regional markets. It further enables stakeholders to identify the key country-level markets within each region.
- Porter's five forces analysis assists stakeholders in assessing the impact of new entrants, competitive rivalry, supplier power, buyer power, and the threat of substitution. It helps stakeholders to analyze the level of competition within the Europe In-Vitro Diagnostics industry and its attractiveness.
- The competitive landscape allows stakeholders to understand their competitive environment and provides insight into the current positions of key players in the market.
This product will be delivered within 5-7 business days.
Table of Contents
Companies Mentioned
- F. Hoffmann-La Roche Ltd
- QIAGEN GmbH
- BioMérieux, Inc.
- Siemens Healthineers AG
- ELITechGroup
- Oncgnostics GmbH
- Abbott Laboratories
- Danaher Corporation
- Sysmex Corporation
- Thermo Fisher Scientific Inc
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 150 |
| Published | September 2024 |
| Forecast Period | 2024 - 2032 |
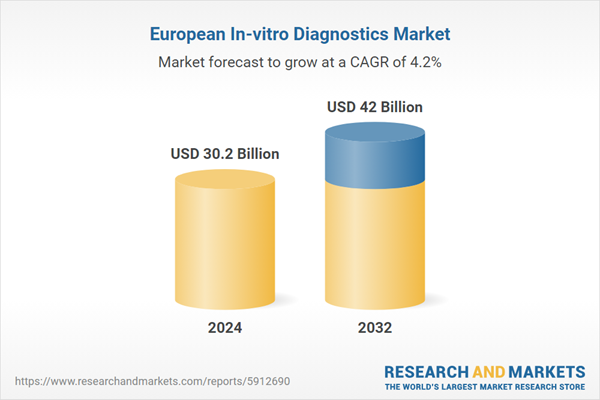
| Estimated Market Value ( USD | $ 30.2 Billion |
| Forecasted Market Value ( USD | $ 42 Billion |
| Compound Annual Growth Rate | 4.2% |
| Regions Covered | Europe |
| No. of Companies Mentioned | 10 |