Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
One of the major drivers of the Global Bio-Pharma Logistics Market is the growing demand for biopharmaceutical products. As advancements in biotechnology and pharmaceutical research lead to the development of more complex and personalized medicines, the need for reliable and efficient logistics services to transport and store these products is paramount. COVID-19 pandemic has underscored the importance of a robust supply chain to ensure the timely delivery of vaccines and therapeutic drugs on a global scale.
Key Market Drivers
Increasing Demand for Biopharmaceutical Products
The increasing demand for biopharmaceutical products is a pivotal factor driving the growth of the Global Bio-Pharma Logistics Market. Biopharmaceuticals, which include vaccines, monoclonal antibodies, gene therapies, and other advanced therapeutic modalities, have gained significant traction in recent years due to their remarkable efficacy in treating a wide range of diseases, including cancer, autoimmune disorders, and infectious diseases.This surge in biopharmaceutical development has catalyzed a corresponding need for efficient and specialized logistics solutions to ensure that these products are manufactured, transported, and distributed seamlessly across the globe. For instance, according to data from the Medical Logistics Branch of the China Federation of Things, the cold vaccine chain market in 2021 was valued at approximately CNY 48.14 billion (USD 7 billion), reflecting a year-on-year growth of 23.3%. In recent years, China has heavily invested in enhancing the development of cold vaccine chains and nationwide traceability systems.
Key Market Challenges
Temperature Control and Monitoring
Temperature control and monitoring pose a significant challenge to the Global Bio-Pharma Logistics Market. Biopharmaceutical products, including vaccines, biologics, gene therapies, and other advanced therapeutics, are highly sensitive to temperature fluctuations. Ensuring that these products are transported and stored under precise temperature conditions is essential to maintain their integrity, safety, and efficacy. The inability to address this challenge effectively can have far-reaching consequences for both the pharmaceutical industry and patient health.One of the primary issues facing the bio-pharma logistics sector is the need for maintaining a consistent temperature throughout the entire supply chain. These products often require stringent temperature control, with variations outside the specified range potentially rendering them ineffective or even dangerous to patients. Achieving this level of temperature control presents several challenges.
The transportation of biopharmaceuticals involves a complex and multifaceted network of providers, including manufacturers, carriers, distribution centers, and healthcare facilities. Each of these entities must adhere to strict temperature requirements to ensure product quality. Any lapse in temperature control at any point along the supply chain can jeopardize the safety and efficacy of the products.
Key Market Trends
Temperature-Controlled Logistics
Temperature-controlled logistics plays a pivotal role in boosting the Global Bio-Pharma Logistics Market. This key trend addresses one of the most critical challenges facing the pharmaceutical and biotechnology sectors: the need to ensure the safe and effective transportation of temperature-sensitive biopharmaceutical products. As the demand for vaccines, biologics, gene therapies, and other advanced therapeutic modalities continues to grow, the necessity for maintaining precise temperature conditions throughout the supply chain has never been more significant. For instance, India ranks among the top 12 biotechnology destinations globally.In 2021, the country witnessed a significant surge in biotech startup registrations, with approximately 1,128 new startups registered - the highest in a single year since 2015. By the end of 2021, the total number of biotechnology startups reached 5,365, with projections suggesting this figure will rise to 10,000 by 2024. India is home to 665 FDA-approved plants in the U.S., accounts for 44% of global abbreviated new drug applications (ANDA), and has over 1,400 manufacturing plants meeting WHO standards. Also, India is the world's third-largest producer of the recombinant Hepatitis B vaccine.
Key Market Players
- Kerry Logistics Network Limited
- Deutsche Post AG
- DHL Group
- Kuehne + Nagel International AG
- UPS (Marken) Inc.
- DB Schenker
- FedEx Corp
- Nippon Express Co Ltd
- World Courier Inc
- SF Express
Report Scope:
In this report, the Global Bio-Pharma Logistics Market has been segmented into the following categories, in addition to the industry trends which have also been detailed below:Bio-Pharma Logistics Market, By Service:
- Transportation
- Warehousing and Distribution
- Value Added Services
Bio-Pharma Logistics Market, By Type of Operation:
- Cold Chain
- Non-cold Chain
Bio-Pharma Logistics Market, By Region:
- North America
- United States
- Canada
- Mexico
- Europe
- France
- United Kingdom
- Italy
- Germany
- Spain
- Asia-Pacific
- China
- India
- Japan
- Australia
- South Korea
- South America
- Brazil
- Argentina
- Colombia
- Middle East & Africa
- South Africa
- Saudi Arabia
- UAE
- Egypt
Competitive Landscape
Company Profiles: Detailed analysis of the major companies present in the Global Bio-Pharma Logistics Market.Available Customizations:
With the given market data, the publisher offers customizations according to a company's specific needs. The following customization options are available for the report.Company Information
- Detailed analysis and profiling of additional market players (up to five).
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
- Kerry Logistics Network Limited
- Deutsche Post AG
- DHL Group
- Kuehne + Nagel International AG
- UPS (Marken) Inc
- DB Schenker
- FedEx Corp.
- Nippon Express Co Ltd
- World Courier Inc.
- SF Express
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 183 |
| Published | March 2025 |
| Forecast Period | 2024 - 2030 |
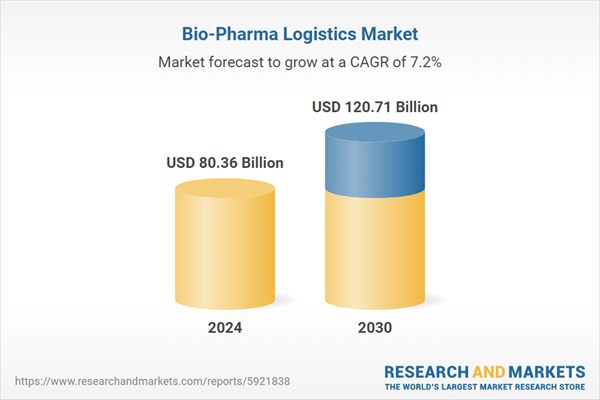
| Estimated Market Value ( USD | $ 80.36 Billion |
| Forecasted Market Value ( USD | $ 120.71 Billion |
| Compound Annual Growth Rate | 7.1% |
| Regions Covered | Global |
| No. of Companies Mentioned | 10 |