Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
In the realm of biomanufacturing, automation emerges as a game-changer, augmenting efficiency and minimizing human error. Additionally, artificial intelligence (AI) lends its expertise in areas such as data analysis, predictive modeling, and optimizing manufacturing processes, further enhancing the overall productivity of the industry.
Despite the remarkable progress in this field, several challenges persist. Regulatory hurdles, the high cost of therapies, and the intricacies of manufacturing pose significant obstacles that necessitate careful attention to ensure sustainable market development and accessibility.
The rapid growth of the APAC Cell and Gene Therapy Biomanufacturing Market is a testament to the region's unwavering commitment to advancing healthcare and its embrace of innovative technologies. With ongoing research and development activities, the APAC region is poised to emerge as a major global player in the dynamic and promising field of cell and gene therapy.
Key Market Drivers
Growing Prevalence of Chronic Diseases
Chronic diseases such as cancer, diabetes, cardiovascular disorders, and neurodegenerative diseases pose a significant health concern in the APAC region. The World Health Organization highlights that non-communicable diseases, predominantly chronic in nature, account for a staggering 80% of all deaths in the Western Pacific region. This alarming trend underscores the pressing need for innovative and effective treatments, thereby propelling the growth of the cell and gene therapy biomanufacturing market.Cell and gene therapies (CGTs) emerge as a revolutionary approach to address these chronic diseases comprehensively. By precisely modifying a patient's genetic material, CGTs hold the potential to not only manage symptoms but also fundamentally alter disease progression, and in some cases, even effect cures. The transformative promise of CGTs has garnered immense attention and interest within the medical community and beyond.
Biomanufacturing of CGTs is an intricate and multifaceted process that demands substantial investment in research and development, highly specialized equipment, and a skilled workforce. Recognizing the immense potential of cell and gene therapies, the APAC region has demonstrated a steadfast commitment to this burgeoning field. Countries like China and South Korea have made substantial investments in CGT research and biomanufacturing infrastructure, positioning themselves as leaders in this transformative domain.
In conclusion, the escalating prevalence of chronic diseases in the APAC region serves as a significant driver for the growth of the Cell and Gene Therapy Biomanufacturing Market. With the potential to revolutionize treatment paradigms, investment and innovation in this sector are poised to flourish. However, realizing the full potential of the market necessitates effective resolutions to regulatory, cost, and technical challenges. By addressing these obstacles, the field can unlock new frontiers of healthcare possibilities and improve patient outcomes.
Surge in Technological Advancements
The Asia Pacific (APAC) Cell and Gene Therapy Biomanufacturing Market is experiencing a robust growth trajectory, propelled by a remarkable surge in technological advancements. These cutting-edge technologies are revolutionizing the development and production of cell and gene therapies (CGTs), offering new avenues for the treatment of a wide range of diseases.One of the significant drivers of this market's expansion is the escalating demand for efficient and scalable cell production. As CGTs become increasingly sophisticated, there is a growing need for innovative methods to produce cells in a more streamlined and scalable manner. Technological innovations, such as advancements in bioreactors, automation, and artificial intelligence (AI), are playing a pivotal role in meeting this demand. These advancements are transforming the biomanufacturing process, making it more efficient, cost-effective, and capable of producing high-quality therapeutic products.
In conclusion, the surge in technological advancements serves as a primary catalyst for the growth of the APAC Cell and Gene Therapy Biomanufacturing Market. As these technologies continue to evolve and mature, they hold immense potential to shape the future of CGT development and production. By unlocking new possibilities and delivering more effective treatments, these advancements promise to significantly improve patient outcomes across a wide spectrum of diseases.
Key Market Challenges
Volatility in Cost of Goods and Pricing Pressures
The Asia Pacific (APAC) Cell and Gene Therapy Biomanufacturing Market has experienced remarkable growth in recent years, propelled by advancements in technology and the rising prevalence of chronic diseases. This flourishing sector, however, is not without its challenges. One of the prominent obstacles is the volatility in the cost of goods and the mounting pressure on pricing.Cell and gene therapies (CGTs) have opened up new horizons in healthcare, offering potentially curative treatments for a wide range of diseases. However, the production and delivery of these therapies entail high levels of personalization, which result in significant costs. Moreover, the steep price tags associated with CGTs have faced criticism from payers. The complex biomanufacturing processes, specialized equipment requirements, and the need for highly skilled personnel contribute to the elevated costs. Consequently, market players are under considerable pressure to lower prices, driven by governments and payers.
Additionally, the biotechnology sector's rapid evolution introduces a significant challenge in terms of cost volatility. For instance, the search for cost reductions is intensified due to Big Pharma's limited recent R&D productivity. This unpredictability in production costs and overall financial planning poses a challenge for companies in the APAC cell and gene therapy biomanufacturing market.
As the APAC cell and gene therapy biomanufacturing market continues to evolve, navigating these challenges becomes crucial for sustainable growth and success in this dynamic industry.
Key Market Trends
Expansion of Contract Development and Manufacturing Organizations (CDMOs)Contract Development and Manufacturing Organizations (CDMOs) have emerged as key players in the biopharmaceutical industry, offering a wide range of services to support the development and production of Cell and Gene Therapies (CGTs). These services encompass crucial aspects such as process development, assay development, and cell therapy manufacturing. By partnering with CDMOs, pharmaceutical companies can tap into their specialized expertise and resources, enabling accelerated development and production of CGTs.
The demand for CDMO services in the CGT sector has witnessed a significant surge in recent years. This upward trend translates into exciting opportunities for CDMO staff, who now have greater prospects for career growth and international advancement. Moreover, the rising demand for CDMOs is not only beneficial for the organizations themselves but also contributes to the overall growth of the APAC Cell and Gene Therapy Biomanufacturing Market.
Interestingly, the product segment of the market is anticipated to be dominated by consumables, with Contract Manufacturing Organizations (CMOs) expected to play a significant role. This highlights the pivotal position of CDMOs in the biomanufacturing ecosystem, as they facilitate the production of essential consumables and provide critical manufacturing support.
However, it is crucial to acknowledge that the expansion of CDMOs also brings along certain challenges. Regulatory compliance remains a top priority, requiring CDMOs to adhere to stringent guidelines to ensure the safety and efficacy of CGTs. Additionally, managing complex supply chains and keeping pace with rapid technological advancements pose ongoing challenges that need to be addressed effectively.
Segmental Insights
Equipment Insights
Based on the category of equipment, the bioreactors/fermenters segment emerged as the dominant player in the Asia Pacific market for cell and gene therapy biomanufacturing in 2022. Bioreactors and fermenters play a crucial role in the manufacturing of cell and gene therapies (CGTs), offering controlled environments that facilitate the cultivation of cells or microorganisms used in the production of these advanced therapies. By providing the necessary conditions for optimal growth and development, bioreactors and fermenters contribute to faster process development, higher yields and quality, and ultimately, reduced production costs. This enables researchers and manufacturers to meet the increasing demand for CGTs while ensuring consistent and efficient production.Application Insights
The formulation and hydration segment is projected to experience rapid growth during the forecast period. Formulation, in the context of cell and gene therapies (CGTs), is a meticulous process that involves combining various components to create a final product suitable for patient administration. This intricate process ensures the stability and efficacy of the therapeutic product.CGTs are highly complex and sensitive, requiring specialized formulation strategies to maintain their viability and functionality. For instance, the formulation must shield the therapeutic product from degradation during storage and transportation, guaranteeing its stability until it reaches the patient. This aspect becomes particularly crucial in the APAC region, where diverse geographical and climatic conditions can potentially impact the stability of CGTs.
Moreover, hydration plays a pivotal role in the biomanufacturing of CGTs. It contributes to several stages of the production process, most notably in cell culture, where it fosters optimal conditions for cell growth and proliferation.
Additionally, hydration holds significance in the formulation process itself. For example, lyophilized (freeze-dried) products necessitate rehydration before administration. This rehydration process demands careful control to preserve the stability and efficacy of the final product. By ensuring proper hydration, the formulation process maximizes the therapeutic potential of CGTs, offering enhanced patient outcomes.
Regional Insights
China emerged as the dominant player in the Asia Pacific Cell and Gene Therapy Biomanufacturing Market in 2022, holding the largest market share in terms of value. China has emerged as a key player in the field of biotechnology, making substantial investments in areas such as gene and cellular therapy. With a strategic focus on "innovation mercantilism," China adopts policies and regulations to foster domestic innovation and reduce reliance on foreign technologies. This approach has yielded remarkable progress in various subfields of health biotechnology, notably in the realms of therapeutic antibodies and gene research.In China, the biotechnology sector is predominantly driven by biologics, encompassing cell and gene therapies. Recognizing the immense potential of these therapies, the government has been actively funding the development of novel cell lines and facilitating collaborations among biotech companies. Such initiatives have created a highly conducive environment for the rapid growth of the cell and gene therapy biomanufacturing market, positioning China as a frontrunner in this domain.
Report Scope:
In this report, the Asia Pacific Cell and Gene Therapy Biomanufacturing Market has been segmented into the following categories, in addition to the industry trends which have also been detailed below:Asia Pacific Cell and Gene Therapy Biomanufacturing Market, By Equipment:
- Bioreactors/Fermenters
- Mixing Systems
- Cell Counters
- Cell Sorters
- Centrifuges
- Incubators
- Biosafety Cabinets
- Freezers
- PCR Systems
- Transfection Systems
- Storage Tanks
- Others
Asia Pacific Cell and Gene Therapy Biomanufacturing Market, By Application:
- Formulation and Hydration
- Cell Culture Processing
- Separation and Filtration
- Others
Asia Pacific Cell and Gene Therapy Biomanufacturing Market, By Country:
- China
- Japan
- South Korea
- Australia
- India
- Rest of Asia-Pacific
Competitive Landscape
Company Profiles: Detailed analysis of the major companies present in the Asia Pacific Cell and Gene Therapy Biomanufacturing Market.Available Customizations:
Asia Pacific Cell and Gene Therapy Biomanufacturing Market report with the given Market data, the publisher offers customizations according to a company's specific needs.This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
- Becton, Dickinson and Company
- Bio-Rad Laboratories, Inc.
- Bio-Techne Corporation
- Danaher Corporation
- Endress+Hauser Group Services AG (Analytik Jena GmbH)
- General Electric Company
- Getinge AB
- Infors AG
- Lonza Group Ltd
- Merck KGaA
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 122 |
| Published | November 2023 |
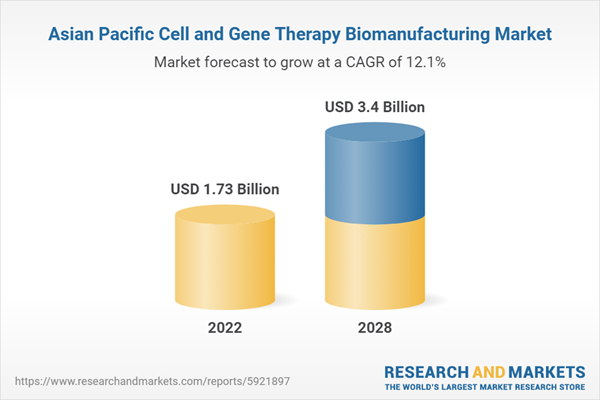
| Forecast Period | 2022 - 2028 |
| Estimated Market Value ( USD | $ 1.73 Billion |
| Forecasted Market Value ( USD | $ 3.4 Billion |
| Compound Annual Growth Rate | 12.1% |
| Regions Covered | Asia Pacific |
| No. of Companies Mentioned | 10 |