Increasing regulatory approvals of innovative therapies is predicted to boost the market growth during the forecast period. An insufficient count of healthy red blood cells is an indicator of anemia. Red blood cells transport oxygen to tissues in the body, and their formation depends on the presence of iron. A low hemoglobin level signifies that the body is not receiving an adequate supply of oxygen. Thus, maintaining a healthy iron level is crucial for overall health. As a result, market participants are directing their efforts toward creating innovative therapies, thus driving the growth of the market. For instance, in March 2022, The Drug Controller General of India has granted approval for Zydus's New Drug Application (NDA) for Oxemia. Oxemia is an oral, small molecule hypoxia-inducible factor-prolyl hydroxylase (HIF-PH) inhibitor and is intended for the treatment of anemia linked with chronic kidney disease (CKD) in India.
By therapy type, parenteral iron therapy was the highest revenue-grossing segment in the global iron deficiency anemia therapy market in 2023 owing to the continual progress in parenteral formulations and the introduction of new products by major companies have significantly enhanced patient convenience and safety. This has made parenteral administration a preferred choice for both healthcare providers and patients. For instance, in May 2023, Emcure Pharmaceuticals has unveiled Orofer FCM 750, an expansion of its parenteral iron product line that includes ferric carboxymaltose (FCM). This new dosage variant is designed to provide a more efficient and convenient solution for patients dealing with iron deficiency anemia (IDA). The FCM, approved by the Drug Controller General of India (DCGI), is specifically indicated for treating iron deficiency when oral preparations are ineffective or unsuitable for use. Additionally, oral iron therapy is predicted to grow at fastest CAGR during the forecast period owing to its convenience, effectiveness, patient preference, widespread availability, the growing introduction of cost-effective products by leading companies, and market participants are addressing the demands of patients in search of efficient and convenient treatments for severe iron deficiency by offering safe and user-friendly oral formulations.
By age group, adult was the highest revenue-grossing segment in the global iron deficiency anemia therapy market in 2023 owing to the prevalence of a hectic & demanding lifestyle, rising incidence of anemia among women of reproductive age, increasing stress & busy lifestyles experienced by young women can exacerbate menstrual irregularities, leading to heavier blood loss & an elevated risk of developing iron deficiency anemia, surge in approvals by regulatory authorities. For instance, in September 2022, Zydus Lifesciences has secured the rights from Pharmacosmos to market the drug MonoFerric (iron isomaltoside) injection in India and Nepal. This drug is intended for the treatment of iron deficiency in adult patients. Additionally, pediatric is anticipated to grow at fastest CAGR during the forecast period. Children are at a higher risk of developing iron deficiency anemia due to their rapid growth, inadequate dietary iron intake, and occasional difficulties in absorbing iron effectively. Furthermore, favorable government initiatives are expected to continue bolstering the growth of this segment in the forecast period.
By end-user, hospitals was the highest revenue-grossing segment in the global iron deficiency anemia therapy market in 2023. Hospitals are well-equipped to manage complex cases, with the necessary infrastructure, medical personnel, and specialized knowledge to handle patients with severe conditions, making them the preferred choice for managing acute and critical cases of IDA. Furthermore, patients who are already hospitalized for other medical conditions may also require concurrent iron therapy. Hospitals can readily incorporate IDA treatment into their overall treatment strategies, ensuring patients receive comprehensive care. They also have access to a broad selection of intravenous formulations, including the latest and most advanced products, providing healthcare providers with increased flexibility to personalize treatments according to the specific needs of each patient. Additionally, home healthcare is predicted to grow at fastest CAGR during the forecast period as it is more cost-effective than hospitalization, especially for patients in need of prolonged or ongoing iron therapy, increasing progress in telehealth & remote monitoring technologies allows healthcare providers to remotely monitor and manage patients' conditions, rendering home healthcare a feasible & effective choice for many individuals, rising emphasis on the creation of user-friendly & safe home administration methods, including subcutaneous iron infusion systems, and growing introduction of oral iron drugs. For instance, in March 2023, FeraMAX Pd Maintenance 45 has been made available in the Canadian market by BioSyent Pharma Inc. This marks the company's third product that employs the patented Polydextrose-Iron Complex as a delivery system for oral iron supplements.
North America region is anticipated for the highest revenue share during the forecast period owing to the growing research & development activities with improved research facilities, the presence of key market players, surge in new product launches, and increasing regulatory approvals. For instance, in June 2023, The U.S. Food and Drug Administration (FDA) has granted approval to Daiichi Sankyo's INJECTAFER for the treatment of iron deficiency in adult patients with heart failure, particularly those classified as New York Heart Association class II/III, with the goal of improving exercise capacity. Daiichi Sankyo noted that this marks the first and only FDA approval for an intravenous iron replacement therapy specifically designed for the treatment of progressive and chronic heart failure in adult patients. Additionally, Asia Pacific region is predicted to grow at fastest CAGR during the forecast period owing to the increasing incidence of the IDA disease, growing awareness among people, surge in favorable strategic initiatives undertaken by market players, rising focus on testing for anemia and initiating treatment from a young age, and increasing introduction of new products. For instance, in March 2023, In Japan, a new intravenous medication called MonoVer for iron deficiency anemia has been introduced by Nippon Shinyaku Co., Ltd. This formulation comprises ferric derisomaltose, known for its high-stability matrix structure binding with iron, and a low immunogenicity linear oligosaccharide (derisomaltose) as the active pharmaceutical ingredient (API). MonoVer provides an effective and dependable solution for addressing iron deficiency anemia through intravenous injection, catering to the distinct requirements of patients in the Japanese market.
Segmentation: Iron Deficiency Anemia Therapy Market Report 2023 - 2034
Iron Deficiency Anemia Therapy Market Analysis & Forecast by Therapy Type 2023 - 2034 (Revenue USD Bn)
- Red Blood Cell Transfusion
- Oral Iron Therapy
- Parenteral Iron Therapy
- Others
Iron Deficiency Anemia Therapy Market Analysis & Forecast by Age Group 2023 - 2034 (Revenue USD Bn)
- Pediatric
- Adults
- Geriatric
Iron Deficiency Anemia Therapy Market Analysis & Forecast by End-user 2023 - 2034 (Revenue USD Bn)
- Hospitals
- Home Healthcare
- Clinics
Iron Deficiency Anemia Therapy Market Analysis & Forecast by Region 2023 - 2034 (Revenue USD Bn)
- North America
- U.S.
- Canada
- Europe
- Germany
- France
- UK
- Spain
- Italy
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- Australia
- South Korea
- Rest of APAC
- Latin America
- Brazil
- Mexico
- Argentina
- Rest of LATAM
- Middle East & Africa
- South Africa
- GCC
- Rest of MEA
Table of Contents
Companies Mentioned
- AbbVie Inc. (Allergan)
- Bayer AG
- CSL Vifor
- Sanofi
- Teoxane
- Johnson & Johnson Services Inc.
- Akebia Therapeutics Inc.
- GSK plc
- AdvaCare Pharma
- Novartis AG
- Apotex Inc.
- Zydus Group
- PHARMACOSMOS A/S
- Covis Pharma GmbH
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 200 |
| Published | January 2024 |
| Forecast Period | 2023 - 2034 |
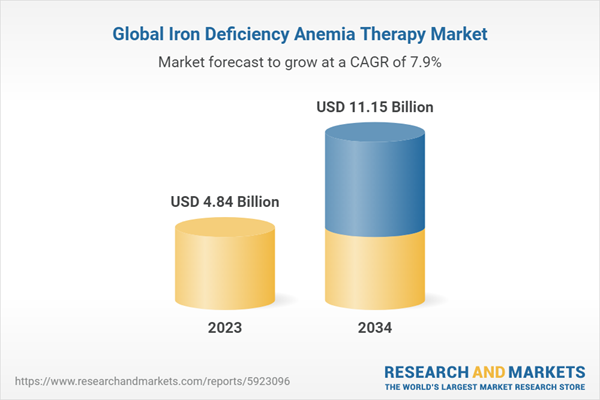
| Estimated Market Value ( USD | $ 4.84 Billion |
| Forecasted Market Value ( USD | $ 11.15 Billion |
| Compound Annual Growth Rate | 7.8% |
| Regions Covered | Global |
| No. of Companies Mentioned | 14 |