1. Research Methodology
1.1. Study Objectives
1.2. Study Scope
1.3. Research Assumptions
1.4. Research Framework
2. Introduction
2.1. Market Definition
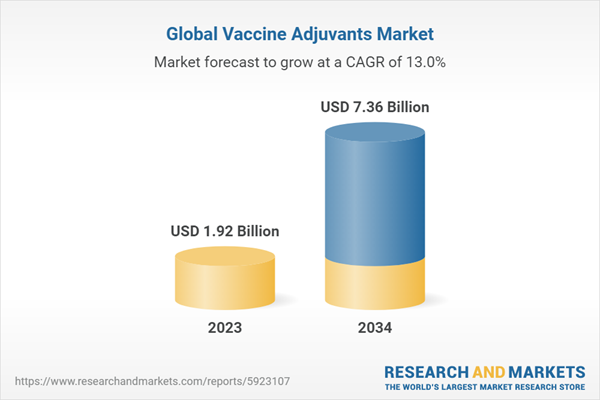
2.2. Global Vaccine Adjuvants Market Overview
4. Market Environment Analysis
4.1. Porter’s 5 Forces Analysis
4.2. PESTEL Analysis
4.3. SWOT Analysis
5. Market Dynamics
5.1. Drivers Analysis
5.2. Restraints Analysis
5.3. Opportunities Analysis
5.4. Threats Analysis
5.5. Trend Analysis
7. Vaccine Adjuvants Market: Product Estimates & Trend Analysis
7.1. Product Segment Opportunity Analysis
7.2. Liposome Adjuvants
7.2.1. Liposome Adjuvants Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
7.3. Mineral Salt-based Adjuvant
7.3.1. Mineral Salt-based Adjuvant Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
7.4. Carbohydrate Adjuvants
7.4.1. Carbohydrate Adjuvants Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
7.5. Adjuvant Emulsions
7.5.1. Adjuvant Emulsions Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
7.6. Tensoactive Adjuvants
7.6.1. Tensoactive Adjuvants Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
7.7. Virus-like Particles (VLP)
7.7.1. Virus-like Particles (VLP) Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
7.8. Bacteria-derived Adjuvants
7.8.1. Bacteria-derived Adjuvants Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
7.9. Others
7.9.1. Others Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
8. Vaccine Adjuvants Market: Usage Estimates & Trend Analysis
8.1. Usage Segment Opportunity Analysis
8.2. Active Immunostimulants
8.2.1. Active Immunostimulants Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
8.3. Vehicle Adjuvants
8.3.1. Vehicle Adjuvants Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
8.4. Carriers
8.4.1. Carriers Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
9. Vaccine Adjuvants Market: Disease Estimates & Trend Analysis
9.1. Disease Segment Opportunity Analysis
9.2. Cancer
9.2.1. Cancer Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
9.3. Infectious Disease
9.3.1. Infectious Disease Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
10. Vaccine Adjuvants Market: Application Estimates & Trend Analysis
10.1. Application Segment Opportunity Analysis
10.2. Commercial Applications
10.2.1. Commercial Applications Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
10.3. Research Applications
10.3.1. Research Applications Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
11. Regional Market Analysis
11.1. Regional Market Opportunity Analysis
12. North America Vaccine Adjuvants Market
12.1. North America Vaccine Adjuvants Market
12.1.1. North America Vaccine Adjuvants Market Size and Forecast, 2023-2034 (Revenue USD Bn)
12.1.2. North America Vaccine Adjuvants Market Size and Forecast, By Country, 2023-2034 (Revenue USD Bn)
12.1.3. North America Vaccine Adjuvants Market Size and Forecast, By Product, 2023-2034 (Revenue USD Bn)
12.1.4. North America Vaccine Adjuvants Market Size and Forecast, By Usage, 2023-2034 (Revenue USD Bn)
12.1.5. North America Vaccine Adjuvants Market Size and Forecast, By Disease, 2023-2034 (Revenue USD Bn)
12.1.6. North America Vaccine Adjuvants Market Size and Forecast, By Application, 2023-2034 (Revenue USD Bn)
12.2. U.S. Global Vaccine Adjuvants Market
12.2.1. U.S. Vaccine Adjuvants Market Size and Forecast, 2023-2034 (Revenue USD Bn)
12.2.2. U.S. Vaccine Adjuvants Market Size and Forecast, By Product, 2023-2034 (Revenue USD Bn)
12.2.3. U.S. Vaccine Adjuvants Market Size and Forecast, By Usage, 2023-2034 (Revenue USD Bn)
12.2.4. U.S. Vaccine Adjuvants Market Size and Forecast, By Disease, 2023-2034 (Revenue USD Bn)
12.2.5. U.S. Vaccine Adjuvants Market Size and Forecast, By Application, 2023-2034 (Revenue USD Bn)
12.3. Canada Global Vaccine Adjuvants Market
12.3.1. Canada Vaccine Adjuvants Market Size and Forecast, 2023-2034 (Revenue USD Bn)
12.3.2. Canada Vaccine Adjuvants Market Size and Forecast, By Product, 2023-2034 (Revenue USD Bn)
12.3.3. Canada Vaccine Adjuvants Market Size and Forecast, By Usage, 2023-2034 (Revenue USD Bn)
12.3.4. Canada Vaccine Adjuvants Market Size and Forecast, By Disease, 2023-2034 (Revenue USD Bn)
12.3.5. Canada Vaccine Adjuvants Market Size and Forecast, By Application, 2023-2034 (Revenue USD Bn)
13. Europe Global Vaccine Adjuvants Market
13.1. Europe Global Vaccine Adjuvants Market
13.1.1. Europe Vaccine Adjuvants Market Size and Forecast, 2023-2034 (Revenue USD Bn)
13.1.2. Europe Vaccine Adjuvants Market Size and Forecast, By Country, 2023-2034 (Revenue USD Bn)
13.1.3. Europe Vaccine Adjuvants Market Size and Forecast, By Product, 2023-2034 (Revenue USD Bn)
13.1.4. Europe Vaccine Adjuvants Market Size and Forecast, By Usage, 2023-2034 (Revenue USD Bn)
13.1.5. Europe Vaccine Adjuvants Market Size and Forecast, By Disease, 2023-2034 (Revenue USD Bn)
13.1.6. Europe Vaccine Adjuvants Market Size and Forecast, By Application, 2023-2034 (Revenue USD Bn)
13.2. Germany Global Vaccine Adjuvants Market
13.2.1. Germany Vaccine Adjuvants Market Size and Forecast, 2023-2034 (Revenue USD Bn)
13.2.2. Germany Vaccine Adjuvants Market Size and Forecast, By Product, 2023-2034 (Revenue USD Bn)
13.2.3. Germany Vaccine Adjuvants Market Size and Forecast, By Usage, 2023-2034 (Revenue USD Bn)
13.2.4. Germany Vaccine Adjuvants Market Size and Forecast, By Disease, 2023-2034 (Revenue USD Bn)
13.2.5. Germany Vaccine Adjuvants Market Size and Forecast, By Application, 2023-2034 (Revenue USD Bn)
13.3. UK Global Vaccine Adjuvants Market
13.3.1. UK Vaccine Adjuvants Market Size and Forecast, 2023-2034 (Revenue USD Bn)
13.3.2. UK Vaccine Adjuvants Market Size and Forecast, By Product, 2023-2034 (Revenue USD Bn)
13.3.3. UK Vaccine Adjuvants Market Size and Forecast, By Usage, 2023-2034 (Revenue USD Bn)
13.3.4. UK Vaccine Adjuvants Market Size and Forecast, By Disease, 2023-2034 (Revenue USD Bn)
13.3.5. UK Vaccine Adjuvants Market Size and Forecast, By Application, 2023-2034 (Revenue USD Bn)
13.4. France Global Vaccine Adjuvants Market
13.4.1. France Vaccine Adjuvants Market Size and Forecast, 2023-2034 (Revenue USD Bn)
13.4.2. France Vaccine Adjuvants Market Size and Forecast, By Product, 2023-2034 (Revenue USD Bn)
13.4.3. France Vaccine Adjuvants Market Size and Forecast, By Usage, 2023-2034 (Revenue USD Bn)
13.4.4. France Vaccine Adjuvants Market Size and Forecast, By Disease, 2023-2034 (Revenue USD Bn)
13.4.5. France Vaccine Adjuvants Market Size and Forecast, By Application, 2023-2034 (Revenue USD Bn)
13.5. Spain Global Vaccine Adjuvants Market
13.5.1. Spain Vaccine Adjuvants Market Size and Forecast, 2023-2034 (Revenue USD Bn)
13.5.2. Spain Vaccine Adjuvants Market Size and Forecast, By Product, 2023-2034 (Revenue USD Bn)
13.5.3. Spain Vaccine Adjuvants Market Size and Forecast, By Usage, 2023-2034 (Revenue USD Bn)
13.5.4. Spain Vaccine Adjuvants Market Size and Forecast, By Disease, 2023-2034 (Revenue USD Bn)
13.5.5. Spain Vaccine Adjuvants Market Size and Forecast, By Application, 2023-2034 (Revenue USD Bn)
13.6. Italy Global Vaccine Adjuvants Market
13.6.1. Italy Vaccine Adjuvants Market Size and Forecast, 2023-2034 (Revenue USD Bn)
13.6.2. Italy Vaccine Adjuvants Market Size and Forecast, By Product, 2023-2034 (Revenue USD Bn)
13.6.3. Italy Vaccine Adjuvants Market Size and Forecast, By Usage, 2023-2034 (Revenue USD Bn)
13.6.4. Italy Vaccine Adjuvants Market Size and Forecast, By Disease, 2023-2034 (Revenue USD Bn)
13.6.5. Italy Vaccine Adjuvants Market Size and Forecast, By Application, 2023-2034 (Revenue USD Bn)
13.7. Rest of Europe Global Vaccine Adjuvants Market
13.7.1. Rest of Europe Vaccine Adjuvants Market Size and Forecast, 2023-2034 (Revenue USD Bn)
13.7.2. Rest of Europe Vaccine Adjuvants Market Size and Forecast, By Product, 2023-2034 (Revenue USD Bn)
13.7.3. Rest of Europe Vaccine Adjuvants Market Size and Forecast, By Usage, 2023-2034 (Revenue USD Bn)
13.7.4. Rest of Europe Vaccine Adjuvants Market Size and Forecast, By Disease, 2023-2034 (Revenue USD Bn)
13.7.5. Rest of Europe Vaccine Adjuvants Market Size and Forecast, By Application, 2023-2034 (Revenue USD Bn)
14. Asia Pacific Global Vaccine Adjuvants Market
14.1. Asia Pacific Global Vaccine Adjuvants Market
14.1.1. Asia Pacific Vaccine Adjuvants Market Size and Forecast, 2023-2034 (Revenue USD Bn)
14.1.2. Asia Pacific Vaccine Adjuvants Market Size and Forecast, By Country, 2023-2034 (Revenue USD Bn)
14.1.3. Asia Pacific Vaccine Adjuvants Market Size and Forecast, By Product, 2023-2034 (Revenue USD Bn)
14.1.4. Asia Pacific Vaccine Adjuvants Market Size and Forecast, By Usage, 2023-2034 (Revenue USD Bn)
14.1.5. Asia Pacific Vaccine Adjuvants Market Size and Forecast, By Disease, 2023-2034 (Revenue USD Bn)
14.1.6. Asia Pacific Vaccine Adjuvants Market Size and Forecast, By Application, 2023-2034 (Revenue USD Bn)
14.2. Japan Global Vaccine Adjuvants Market
14.2.1. Japan Vaccine Adjuvants Market Size and Forecast, 2023-2034 (Revenue USD Bn)
14.2.2. Japan Vaccine Adjuvants Market Size and Forecast, By Product, 2023-2034 (Revenue USD Bn)
14.2.3. Japan Vaccine Adjuvants Market Size and Forecast, By Usage, 2023-2034 (Revenue USD Bn)
14.2.4. Japan Vaccine Adjuvants Market Size and Forecast, By Disease, 2023-2034 (Revenue USD Bn)
14.2.5. Japan Vaccine Adjuvants Market Size and Forecast, By Application, 2023-2034 (Revenue USD Bn)
14.3. China Global Vaccine Adjuvants Market
14.3.1. China Vaccine Adjuvants Market Size and Forecast, 2023-2034 (Revenue USD Bn)
14.3.2. China Vaccine Adjuvants Market Size and Forecast, By Product, 2023-2034 (Revenue USD Bn)
14.3.3. China Vaccine Adjuvants Market Size and Forecast, By Usage, 2023-2034 (Revenue USD Bn)
14.3.4. China Vaccine Adjuvants Market Size and Forecast, By Disease, 2023-2034 (Revenue USD Bn)
14.3.5. China Vaccine Adjuvants Market Size and Forecast, By Application, 2023-2034 (Revenue USD Bn)
14.4. India Global Vaccine Adjuvants Market
14.4.1. India Vaccine Adjuvants Market Size and Forecast, 2023-2034 (Revenue USD Bn)
14.4.2. India Vaccine Adjuvants Market Size and Forecast, By Product, 2023-2034 (Revenue USD Bn)
14.4.3. India Vaccine Adjuvants Market Size and Forecast, By Usage, 2023-2034 (Revenue USD Bn)
14.4.4. India Vaccine Adjuvants Market Size and Forecast, By Disease, 2023-2034 (Revenue USD Bn)
14.4.5. India Vaccine Adjuvants Market Size and Forecast, By Application, 2023-2034 (Revenue USD Bn)
14.5. South Korea Global Vaccine Adjuvants Market
14.5.1. South Korea Vaccine Adjuvants Market Size and Forecast, 2023-2034 (Revenue USD Bn)
14.5.2. South Korea Vaccine Adjuvants Market Size and Forecast, By Product, 2023-2034 (Revenue USD Bn)
14.5.3. South Korea Vaccine Adjuvants Market Size and Forecast, By Usage, 2023-2034 (Revenue USD Bn)
14.5.4. South Korea Vaccine Adjuvants Market Size and Forecast, By Disease, 2023-2034 (Revenue USD Bn)
14.5.5. South Korea Vaccine Adjuvants Market Size and Forecast, By Application, 2023-2034 (Revenue USD Bn)
14.6. Australia Global Vaccine Adjuvants Market
14.6.1. Australia Vaccine Adjuvants Market Size and Forecast, 2023-2034 (Revenue USD Bn)
14.6.2. Australia Vaccine Adjuvants Market Size and Forecast, By Product, 2023-2034 (Revenue USD Bn)
14.6.3. Australia Vaccine Adjuvants Market Size and Forecast, By Usage, 2023-2034 (Revenue USD Bn)
14.6.4. Australia Vaccine Adjuvants Market Size and Forecast, By Disease, 2023-2034 (Revenue USD Bn)
14.6.5. Australia Vaccine Adjuvants Market Size and Forecast, By Application, 2023-2034 (Revenue USD Bn)
14.7. Rest of Asia Pacific Global Vaccine Adjuvants Market
14.7.1. Rest of Asia Pacific Vaccine Adjuvants Market Size and Forecast, 2023-2034 (Revenue USD Bn)
14.7.2. Rest of Asia Pacific Vaccine Adjuvants Market Size and Forecast, By Product, 2023-2034 (Revenue USD Bn)
14.7.3. Rest of Asia Pacific Vaccine Adjuvants Market Size and Forecast, By Usage, 2023-2034 (Revenue USD Bn)
14.7.4. Rest of Asia Pacific Vaccine Adjuvants Market Size and Forecast, By Disease, 2023-2034 (Revenue USD Bn)
14.7.5. Rest of Asia Pacific Vaccine Adjuvants Market Size and Forecast, By Application, 2023-2034 (Revenue USD Bn)
15. Latin America Global Vaccine Adjuvants Market
15.1. Latin America Global Vaccine Adjuvants Market
15.1.1. Latin America Vaccine Adjuvants Market Size and Forecast, 2023-2034 (Revenue USD Bn)
15.1.2. Latin America Vaccine Adjuvants Market Size and Forecast, By Country, 2023-2034 (Revenue USD Bn)
15.1.3. Latin America Vaccine Adjuvants Market Size and Forecast, By Product, 2023-2034 (Revenue USD Bn)
15.1.4. Latin America Vaccine Adjuvants Market Size and Forecast, By Usage, 2023-2034 (Revenue USD Bn)
15.1.5. Latin America Vaccine Adjuvants Market Size and Forecast, By Disease, 2023-2034 (Revenue USD Bn)
15.1.6. Latin America Vaccine Adjuvants Market Size and Forecast, By Application, 2023-2034 (Revenue USD Bn)
15.2. Brazil Global Vaccine Adjuvants Market
15.2.1. Brazil Vaccine Adjuvants Market Size and Forecast, 2023-2034 (Revenue USD Bn)
15.2.2. Brazil Vaccine Adjuvants Market Size and Forecast, By Product, 2023-2034 (Revenue USD Bn)
15.2.3. Brazil Vaccine Adjuvants Market Size and Forecast, By Usage, 2023-2034 (Revenue USD Bn)
15.2.4. Brazil Vaccine Adjuvants Market Size and Forecast, By Disease, 2023-2034 (Revenue USD Bn)
15.2.5. Brazil Vaccine Adjuvants Market Size and Forecast, By Application, 2023-2034 (Revenue USD Bn)
15.3. Mexico Global Vaccine Adjuvants Market
15.3.1. Mexico Vaccine Adjuvants Market Size and Forecast, 2023-2034 (Revenue USD Bn)
15.3.2. Mexico Vaccine Adjuvants Market Size and Forecast, By Product, 2023-2034 (Revenue USD Bn)
15.3.3. Mexico Vaccine Adjuvants Market Size and Forecast, By Usage, 2023-2034 (Revenue USD Bn)
15.3.4. Mexico Vaccine Adjuvants Market Size and Forecast, By Disease, 2023-2034 (Revenue USD Bn)
15.3.5. Mexico Vaccine Adjuvants Market Size and Forecast, By Application, 2023-2034 (Revenue USD Bn)
15.4. Argentina Global Vaccine Adjuvants Market
15.4.1. Argentina Vaccine Adjuvants Market Size and Forecast, 2023-2034 (Revenue USD Bn)
15.4.2. Argentina Vaccine Adjuvants Market Size and Forecast, By Product, 2023-2034 (Revenue USD Bn)
15.4.3. Argentina Vaccine Adjuvants Market Size and Forecast, By Usage, 2023-2034 (Revenue USD Bn)
15.4.4. Argentina Vaccine Adjuvants Market Size and Forecast, By Disease, 2023-2034 (Revenue USD Bn)
15.4.5. Argentina Vaccine Adjuvants Market Size and Forecast, By Application, 2023-2034 (Revenue USD Bn)
15.5. Rest of Latin America Global Vaccine Adjuvants Market
15.5.1. Rest of Latin America Vaccine Adjuvants Market Size and Forecast, 2023-2034 (Revenue USD Bn)
15.5.2. Rest of Latin America Vaccine Adjuvants Market Size and Forecast, By Product, 2023-2034 (Revenue USD Bn)
15.5.3. Rest of Latin America Vaccine Adjuvants Market Size and Forecast, By Usage, 2023-2034 (Revenue USD Bn)
15.5.4. Rest of Latin America Vaccine Adjuvants Market Size and Forecast, By Disease, 2023-2034 (Revenue USD Bn)
15.5.5. Rest of Latin America Vaccine Adjuvants Market Size and Forecast, By Application, 2023-2034 (Revenue USD Bn)
16. MEA Global Vaccine Adjuvants Market
16.1. MEA Global Vaccine Adjuvants Market
16.1.1. MEA Vaccine Adjuvants Market Size and Forecast, 2023-2034 (Revenue USD Bn)
16.1.2. MEA Vaccine Adjuvants Market Size and Forecast, By Country, 2023-2034 (Revenue USD Bn)
16.1.3. MEA Vaccine Adjuvants Market Size and Forecast, By Product, 2023-2034 (Revenue USD Bn)
16.1.4. MEA Vaccine Adjuvants Market Size and Forecast, By Usage, 2023-2034 (Revenue USD Bn)
16.1.5. MEA Vaccine Adjuvants Market Size and Forecast, By Disease, 2023-2034 (Revenue USD Bn)
16.1.6. MEA Vaccine Adjuvants Market Size and Forecast, By Application, 2023-2034 (Revenue USD Bn)
16.2. GCC Global Vaccine Adjuvants Market
16.2.1. GCC Vaccine Adjuvants Market Size and Forecast, 2023-2034 (Revenue USD Bn)
16.2.2. GCC Vaccine Adjuvants Market Size and Forecast, By Product, 2023-2034 (Revenue USD Bn)
16.2.3. GCC Vaccine Adjuvants Market Size and Forecast, By Usage, 2023-2034 (Revenue USD Bn)
16.2.4. GCC Vaccine Adjuvants Market Size and Forecast, By Disease, 2023-2034 (Revenue USD Bn)
16.2.5. GCC Vaccine Adjuvants Market Size and Forecast, By Application, 2023-2034 (Revenue USD Bn)
16.3. South Africa Global Vaccine Adjuvants Market
16.3.1. South Africa Vaccine Adjuvants Market Size and Forecast, 2023-2034 (Revenue USD Bn)
16.3.2. South Africa Vaccine Adjuvants Market Size and Forecast, By Product, 2023-2034 (Revenue USD Bn)
16.3.3. South Africa Vaccine Adjuvants Market Size and Forecast, By Usage, 2023-2034 (Revenue USD Bn)
16.3.4. South Africa Vaccine Adjuvants Market Size and Forecast, By Disease, 2023-2034 (Revenue USD Bn)
16.3.5. South Africa Vaccine Adjuvants Market Size and Forecast, By Application, 2023-2034 (Revenue USD Bn)
16.4. Rest of MEA Global Vaccine Adjuvants Market
16.4.1. Rest of MEA Vaccine Adjuvants Market Size and Forecast, 2023-2034 (Revenue USD Bn)
16.4.2. Rest of MEA Vaccine Adjuvants Market Size and Forecast, By Product, 2023-2034 (Revenue USD Bn)
16.4.3. Rest of MEA Vaccine Adjuvants Market Size and Forecast, By Usage, 2023-2034 (Revenue USD Bn)
16.4.4. Rest of MEA Vaccine Adjuvants Market Size and Forecast, By Disease, 2023-2034 (Revenue USD Bn)
16.4.5. Rest of MEA Vaccine Adjuvants Market Size and Forecast, By Application, 2023-2034 (Revenue USD Bn)
17. Competitor Analysis
17.1. Company Market Share Analysis, 2023
17.2. Major Recent Developments
18. Company Profiles
18.1. Merck & Co. Inc.
18.2. Thermo Fisher Scientific Inc.
18.3. Pacific GeneTech Limited
18.4. Associated British Foods PLC (SPI Pharma Inc.)
18.5. CSL Limited
18.6. OZ Biosciences
18.7. InvivoGen
18.8. Air Liquide (Seppic)
18.9. Dynavax Technologies Corporation
18.10. Croda International PLC
18.11. GlaxoSmithKline PLC
18.12. VertellusAgenus Inc.
18.13. Novavax Inc.
18.14. Adjuvatis
18.15. Aphios Corporation
18.16. Other Prominent Players