Increasing focus on development of therapies & drugs by market players is predicted to boost the market growth during the forecast period. Nasopharyngeal carcinoma is an uncommon form of cancer that originates in the nasopharynx. Symptoms associated with nasopharyngeal carcinoma may include a sore throat, the presence of a lump in the nose or neck, breathing or speaking difficulties, hearing problems, ear pain or ringing, and headaches. Diagnosis of nasopharyngeal cancers involves various techniques like EBV antibody testing, X-rays, ultrasounds, endoscopy, biopsies, CT scans, MRI scans, PET scans, or PET-CT scans. Consequently, companies in the healthcare sector are emphasizing the development of innovative therapies and drugs for this condition. For instance, in July 2022, Junshi Biosciences Co., Ltd. and Coherus BioSciences Inc. have announced that the United States Food and Drug Administration (FDA) has accepted the Biologics License Application (BLA) resubmission for Toripalimab. This application seeks approval for toripalimab in combination with gemcitabine and cisplatin as a first-line treatment for patients with advanced recurrent or metastatic nasopharyngeal carcinoma (NPC), as well as for toripalimab monotherapy in the second-line or later treatment of recurrent or metastatic NPC after prior platinum-containing chemotherapy.
By therapy, chemotherapy was the highest revenue-grossing segment in the global nasopharyngeal cancer market in 2023 owing to rising prevalence of NPC among the target population and surge in product approvals. For instance, in June 2022, The China National Medical Products Administration (NMPA) is expected to approve the use of BeiGene's tislelizumab in combination with chemotherapy as a first-line treatment for patients with recurrent or metastatic nasopharyngeal carcinoma (NPC). Additionally, radiation therapy is predicted to grow at fastest CAGR during the forecast period owing to the increasing research & development activities and growing focus on development of advanced therapies.
By end-users, hospitals & clinics was the highest revenue-grossing segment in the global nasopharyngeal cancer market in 2023 owing to the availability of nasopharyngeal carcinoma treatments & procedures, increasing adoption of technologies, rising investment by major market players to offer effective treatment solutions, aiming to enhance healthcare quality, improve productivity, & ultimately achieve better patient outcomes, and surge in research & development activities. For instance, in January 2022, The Wistar Institute has initiated a phase 2 clinical trial to assess the safety and effectiveness of the oral inhibitor VK-2019 in patients with advanced Epstein-Barr virus-positive nasopharyngeal carcinoma, a rare form of head and neck cancer lymphoma. This trial is being led by researchers at the Stanford University School of Medicine. Additionally, ambulatory surgery centers is predicted to grow at fastest CAGR during the forecast period owing to the increasing number of patients suffering from nasopharyngeal cancer and rising adoption of advanced therapies.
North America region is anticipated for the highest revenue share during the forecast period owing to the increasing prevalence of nasopharyngeal cancer, the presence of leading market players, surge in introduction of new products, and rising number of clinical trial procedures. For instance, in June 2023, Coherus BioSciences, Inc. has unveiled the final overall survival (OS) findings from the JUPITER-02 study, which were presented at the American Society for Clinical Oncology (ASCO) Annual Meeting. The JUPITER-02 study is a Phase 3 clinical trial that is randomized, double-blind, and placebo-controlled. It assesses the use of toripalimab in combination with gemcitabine and cisplatin as a first-line treatment for patients with recurrent or metastatic nasopharyngeal carcinoma (NPC). Additionally, Asia Pacific region is predicted to grow at fastest CAGR during the forecast period owing to the rising awareness regarding the treatment & diagnosis of nasopharyngeal cancer among population, growing technological advances, and increasing initiatives by market players. For instance, in June 2022, KMC clinics in Kolkata, India, have initiated a Cancer Screening Program, which encompasses screening for nasopharyngeal cancer as part of the head and neck division. This screening program has been launched in partnership with the Indian Medical Association (IMA), the Indian Dental Association (IDA), and Medica Superspecialty Hospital. It is anticipated that this program will be conducted at all 16 centers operated by KMC.
Segmentation: Nasopharyngeal Cancer Market Report 2023 - 2034
Nasopharyngeal Cancer Market Analysis & Forecast by Therapy 2023 - 2034 (Revenue USD Bn)
- Immunotherapy
- Chemotherapy
- Radiation Therapy
- Other
Nasopharyngeal Cancer Market Analysis & Forecast by End-user 2023 - 2034 (Revenue USD Bn)
- Ambulatory Surgery Centers
- Hospitals & Clinics
- Other
Nasopharyngeal Cancer Market Analysis & Forecast by Region 2023 - 2034 (Revenue USD Bn)
- North America
- U.S.
- Canada
- Europe
- Germany
- France
- UK
- Spain
- Italy
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- Australia
- South Korea
- Rest of APAC
- Latin America
- Brazil
- Mexico
- Argentina
- Rest of LATAM
- Middle East & Africa
- South Africa
- GCC
- Rest of MEA
Table of Contents
Companies Mentioned
- Eli Lilly and Company
- Pfizer Inc.
- Merck & Co. Inc.
- Fresenius SE & Co. KGaA
- CELGENE CORPORATION
- Cyclacel Pharmaceuticals Inc.
- Sanofi
- F. Hoffmann-La Roche Ltd
- BioDiem Ltd
- Teva Pharmaceutical Industries Ltd.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 200 |
| Published | January 2024 |
| Forecast Period | 2023 - 2034 |
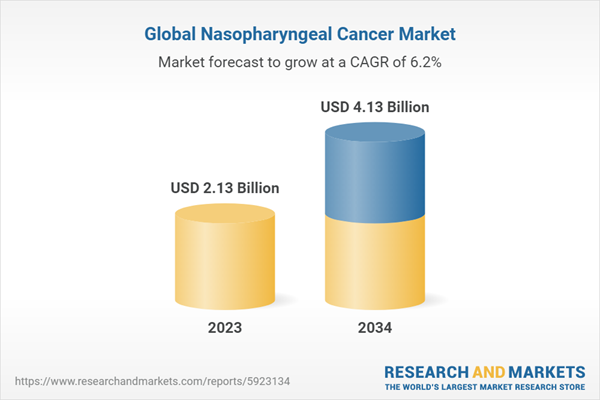
| Estimated Market Value ( USD | $ 2.13 Billion |
| Forecasted Market Value ( USD | $ 4.13 Billion |
| Compound Annual Growth Rate | 6.2% |
| Regions Covered | Global |
| No. of Companies Mentioned | 10 |