1. Research Methodology
1.1. Study Objectives
1.2. Study Scope
1.3. Research Assumptions
1.4. Research Framework
2. Introduction
2.1. Market Definition
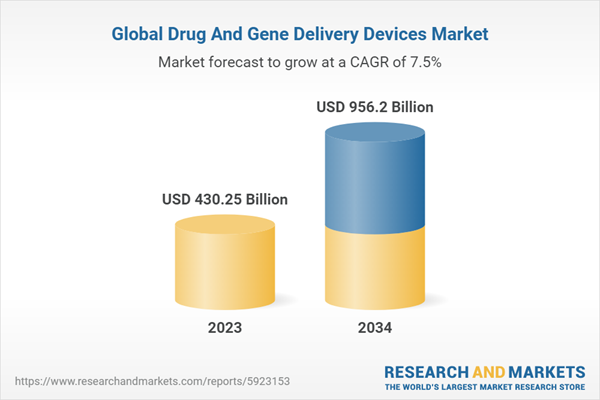
2.2. Global Drug And Gene Delivery Devices Market Overview
4. Market Environment Analysis
4.1. Porter’s 5 Forces Analysis
4.2. PESTEL Analysis
4.3. SWOT Analysis
5. Market Dynamics
5.1. Drivers Analysis
5.2. Restraints Analysis
5.3. Opportunities Analysis
5.4. Threats Analysis
5.5. Trend Analysis
7. Drug And Gene Delivery Devices Market: Product Estimates & Trend Analysis
7.1. Product Segment Opportunity Analysis
7.2. Intravenous Catheter
7.2.1. Intravenous Catheter Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
7.3. Infusion Bags
7.3.1. Infusion Bags Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
7.4. Subretinal Injection Cannula
7.4.1. Subretinal Injection Cannula Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
7.5. Sterile Insulin Syringe
7.5.1. Sterile Insulin Syringe Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
7.6. Extension Tube
7.6.1. Extension Tube Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
7.7. Pre-filled Syringe
7.7.1. Pre-filled Syringe Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
8. Drug And Gene Delivery Devices Market: Commercialized Drugs Estimates & Trend Analysis
8.1. Commercialized Drugs Segment Opportunity Analysis
8.2. Kymriah (Drug Delivery Devices)
8.2.1. Kymriah (Drug Delivery Devices) Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
8.3. Zolgensma (Drug Delivery Devices)
8.3.1. Zolgensma (Drug Delivery Devices) Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
8.4. Yescarta (Drug Delivery Devices)
8.4.1. Yescarta (Drug Delivery Devices) Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
8.5. Provenge (Drug Delivery Devices)
8.5.1. Provenge (Drug Delivery Devices) Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
8.6. Luxturna (Drug Delivery Devices)
8.6.1. Luxturna (Drug Delivery Devices) Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
8.7. Strimvelis (Drug Delivery Devices)
8.7.1. Strimvelis (Drug Delivery Devices) Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
9. Drug And Gene Delivery Devices Market: Methods Estimates & Trend Analysis
9.1. Methods Segment Opportunity Analysis
9.2. Ex vivo
9.2.1. Ex vivo Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
9.3. In situ
9.3.1. In situ Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
10. Drug And Gene Delivery Devices Market: Vector Estimates & Trend Analysis
10.1. Vector Segment Opportunity Analysis
10.2. Non-viral
10.2.1. Non-viral Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
10.3. Viral
10.3.1. Viral Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
11. Drug And Gene Delivery Devices Market: Route of Administration Estimates & Trend Analysis
11.1. Vector Segment Opportunity Analysis
11.2. Transdermal
11.2.1. Transdermal Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
11.3. Oral
11.3.1. Oral Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
11.4. Topical
11.4.1. Topical Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
11.5. Injectable
11.5.1. Injectable Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
11.6. Nasal
11.6.1. Nasal Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
11.7. Ocular
11.7.1. Ocular Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
11.8. Inhalation
11.8.1. Inhalation Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
12. Regional Market Analysis
12.1. Regional Market Opportunity Analysis
13. North America Drug And Gene Delivery Devices Market
13.1. North America Drug And Gene Delivery Devices Market
13.1.1. North America Drug And Gene Delivery Devices Market Size and Forecast, 2023-2034 (Revenue USD Bn)
13.1.2. North America Drug And Gene Delivery Devices Market Size and Forecast, By Country, 2023-2034 (Revenue USD Bn)
13.1.3. North America Drug And Gene Delivery Devices Market Size and Forecast, By Product, 2023-2034 (Revenue USD Bn)
13.1.4. North America Drug And Gene Delivery Devices Market Size and Forecast, By Commercialized Drugs, 2023-2034 (Revenue USD Bn)
13.1.5. North America Drug And Gene Delivery Devices Market Size and Forecast, By Methods, 2023-2034 (Revenue USD Bn)
13.1.6. North America Drug And Gene Delivery Devices Market Size and Forecast, By Vector, 2023-2034 (Revenue USD Bn)
13.1.7. North America Drug And Gene Delivery Devices Market Size and Forecast, By Route of Administration, 2023-2034 (Revenue USD Bn)
13.2. U.S. Global Drug And Gene Delivery Devices Market
13.2.1. U.S. Drug And Gene Delivery Devices Market Size and Forecast, 2023-2034 (Revenue USD Bn)
13.2.2. U.S. Drug And Gene Delivery Devices Market Size and Forecast, By Product, 2023-2034 (Revenue USD Bn)
13.2.3. U.S. Drug And Gene Delivery Devices Market Size and Forecast, By Commercialized Drugs, 2023-2034 (Revenue USD Bn)
13.2.4. U.S. Drug And Gene Delivery Devices Market Size and Forecast, By Methods, 2023-2034 (Revenue USD Bn)
13.2.5. U.S. Drug And Gene Delivery Devices Market Size and Forecast, By Vector, 2023-2034 (Revenue USD Bn)
13.2.6. U.S. Drug And Gene Delivery Devices Market Size and Forecast, By Route of Administration, 2023-2034 (Revenue USD Bn)
13.3. Canada Global Drug And Gene Delivery Devices Market
13.3.1. Canada Drug And Gene Delivery Devices Market Size and Forecast, 2023-2034 (Revenue USD Bn)
13.3.2. Canada Drug And Gene Delivery Devices Market Size and Forecast, By Product, 2023-2034 (Revenue USD Bn)
13.3.3. Canada Drug And Gene Delivery Devices Market Size and Forecast, By Commercialized Drugs, 2023-2034 (Revenue USD Bn)
13.3.4. Canada Drug And Gene Delivery Devices Market Size and Forecast, By Methods, 2023-2034 (Revenue USD Bn)
13.3.5. Canada Drug And Gene Delivery Devices Market Size and Forecast, By Vector, 2023-2034 (Revenue USD Bn)
13.3.6. Canada Drug And Gene Delivery Devices Market Size and Forecast, By Route of Administration, 2023-2034 (Revenue USD Bn)
14. Europe Global Drug And Gene Delivery Devices Market
14.1. Europe Global Drug And Gene Delivery Devices Market
14.1.1. Europe Drug And Gene Delivery Devices Market Size and Forecast, 2023-2034 (Revenue USD Bn)
14.1.2. Europe Drug And Gene Delivery Devices Market Size and Forecast, By Country, 2023-2034 (Revenue USD Bn)
14.1.3. Europe Drug And Gene Delivery Devices Market Size and Forecast, By Product, 2023-2034 (Revenue USD Bn)
14.1.4. Europe Drug And Gene Delivery Devices Market Size and Forecast, By Commercialized Drugs, 2023-2034 (Revenue USD Bn)
14.1.5. Europe Drug And Gene Delivery Devices Market Size and Forecast, By Methods, 2023-2034 (Revenue USD Bn)
14.1.6. Europe Drug And Gene Delivery Devices Market Size and Forecast, By Vector, 2023-2034 (Revenue USD Bn)
14.1.7. Europe Drug And Gene Delivery Devices Market Size and Forecast, By Route of Administration, 2023-2034 (Revenue USD Bn)
14.2. Germany Global Drug And Gene Delivery Devices Market
14.2.1. Germany Drug And Gene Delivery Devices Market Size and Forecast, 2023-2034 (Revenue USD Bn)
14.2.2. Germany Drug And Gene Delivery Devices Market Size and Forecast, By Product, 2023-2034 (Revenue USD Bn)
14.2.3. Germany Drug And Gene Delivery Devices Market Size and Forecast, By Commercialized Drugs, 2023-2034 (Revenue USD Bn)
14.2.4. Germany Drug And Gene Delivery Devices Market Size and Forecast, By Methods, 2023-2034 (Revenue USD Bn)
14.2.5. Germany Drug And Gene Delivery Devices Market Size and Forecast, By Vector, 2023-2034 (Revenue USD Bn)
14.2.6. Germany Drug And Gene Delivery Devices Market Size and Forecast, By Route of Administration, 2023-2034 (Revenue USD Bn)
14.3. UK Global Drug And Gene Delivery Devices Market
14.3.1. UK Drug And Gene Delivery Devices Market Size and Forecast, 2023-2034 (Revenue USD Bn)
14.3.2. UK Drug And Gene Delivery Devices Market Size and Forecast, By Product, 2023-2034 (Revenue USD Bn)
14.3.3. UK Drug And Gene Delivery Devices Market Size and Forecast, By Commercialized Drugs, 2023-2034 (Revenue USD Bn)
14.3.4. UK Drug And Gene Delivery Devices Market Size and Forecast, By Methods, 2023-2034 (Revenue USD Bn)
14.3.5. UK Drug And Gene Delivery Devices Market Size and Forecast, By Vector, 2023-2034 (Revenue USD Bn)
14.3.6. UK Drug And Gene Delivery Devices Market Size and Forecast, By Route of Administration, 2023-2034 (Revenue USD Bn)
14.4. France Global Drug And Gene Delivery Devices Market
14.4.1. France Drug And Gene Delivery Devices Market Size and Forecast, 2023-2034 (Revenue USD Bn)
14.4.2. France Drug And Gene Delivery Devices Market Size and Forecast, By Product, 2023-2034 (Revenue USD Bn)
14.4.3. France Drug And Gene Delivery Devices Market Size and Forecast, By Commercialized Drugs, 2023-2034 (Revenue USD Bn)
14.4.4. France Drug And Gene Delivery Devices Market Size and Forecast, By Methods, 2023-2034 (Revenue USD Bn)
14.4.5. France Drug And Gene Delivery Devices Market Size and Forecast, By Vector, 2023-2034 (Revenue USD Bn)
14.4.6. France Drug And Gene Delivery Devices Market Size and Forecast, By Route of Administration, 2023-2034 (Revenue USD Bn)
14.5. Spain Global Drug And Gene Delivery Devices Market
14.5.1. Spain Drug And Gene Delivery Devices Market Size and Forecast, 2023-2034 (Revenue USD Bn)
14.5.2. Spain Drug And Gene Delivery Devices Market Size and Forecast, By Product, 2023-2034 (Revenue USD Bn)
14.5.3. Spain Drug And Gene Delivery Devices Market Size and Forecast, By Commercialized Drugs, 2023-2034 (Revenue USD Bn)
14.5.4. Spain Drug And Gene Delivery Devices Market Size and Forecast, By Methods, 2023-2034 (Revenue USD Bn)
14.5.5. Spain Drug And Gene Delivery Devices Market Size and Forecast, By Vector, 2023-2034 (Revenue USD Bn)
14.5.6. Spain Drug And Gene Delivery Devices Market Size and Forecast, By Route of Administration, 2023-2034 (Revenue USD Bn)
14.6. Italy Global Drug And Gene Delivery Devices Market
14.6.1. Italy Drug And Gene Delivery Devices Market Size and Forecast, 2023-2034 (Revenue USD Bn)
14.6.2. Italy Drug And Gene Delivery Devices Market Size and Forecast, By Product, 2023-2034 (Revenue USD Bn)
14.6.3. Italy Drug And Gene Delivery Devices Market Size and Forecast, By Commercialized Drugs, 2023-2034 (Revenue USD Bn)
14.6.4. Italy Drug And Gene Delivery Devices Market Size and Forecast, By Methods, 2023-2034 (Revenue USD Bn)
14.6.5. Italy Drug And Gene Delivery Devices Market Size and Forecast, By Vector, 2023-2034 (Revenue USD Bn)
14.6.6. Italy Drug And Gene Delivery Devices Market Size and Forecast, By Route of Administration, 2023-2034 (Revenue USD Bn)
14.7. Rest of Europe Global Drug And Gene Delivery Devices Market
14.7.1. Rest of Europe Drug And Gene Delivery Devices Market Size and Forecast, 2023-2034 (Revenue USD Bn)
14.7.2. Rest of Europe Drug And Gene Delivery Devices Market Size and Forecast, By Product, 2023-2034 (Revenue USD Bn)
14.7.3. Rest of Europe Drug And Gene Delivery Devices Market Size and Forecast, By Commercialized Drugs, 2023-2034 (Revenue USD Bn)
14.7.4. Rest of Europe Drug And Gene Delivery Devices Market Size and Forecast, By Methods, 2023-2034 (Revenue USD Bn)
14.7.5. Rest of Europe Drug And Gene Delivery Devices Market Size and Forecast, By Vector, 2023-2034 (Revenue USD Bn)
14.7.6. Rest of Europe Drug And Gene Delivery Devices Market Size and Forecast, By Route of Administration, 2023-2034 (Revenue USD Bn)
15. Asia Pacific Global Drug And Gene Delivery Devices Market
15.1. Asia Pacific Global Drug And Gene Delivery Devices Market
15.1.1. Asia Pacific Drug And Gene Delivery Devices Market Size and Forecast, 2023-2034 (Revenue USD Bn)
15.1.2. Asia Pacific Drug And Gene Delivery Devices Market Size and Forecast, By Country, 2023-2034 (Revenue USD Bn)
15.1.3. Asia Pacific Drug And Gene Delivery Devices Market Size and Forecast, By Product, 2023-2034 (Revenue USD Bn)
15.1.4. Asia Pacific Drug And Gene Delivery Devices Market Size and Forecast, By Commercialized Drugs, 2023-2034 (Revenue USD Bn)
15.1.5. Asia Pacific Drug And Gene Delivery Devices Market Size and Forecast, By Methods, 2023-2034 (Revenue USD Bn)
15.1.6. Asia Pacific Drug And Gene Delivery Devices Market Size and Forecast, By Vector, 2023-2034 (Revenue USD Bn)
15.1.7. Asia Pacific Drug And Gene Delivery Devices Market Size and Forecast, By Route of Administration, 2023-2034 (Revenue USD Bn)
15.2. Japan Global Drug And Gene Delivery Devices Market
15.2.1. Japan Drug And Gene Delivery Devices Market Size and Forecast, 2023-2034 (Revenue USD Bn)
15.2.2. Japan Drug And Gene Delivery Devices Market Size and Forecast, By Product, 2023-2034 (Revenue USD Bn)
15.2.3. Japan Drug And Gene Delivery Devices Market Size and Forecast, By Commercialized Drugs, 2023-2034 (Revenue USD Bn)
15.2.4. Japan Drug And Gene Delivery Devices Market Size and Forecast, By Methods, 2023-2034 (Revenue USD Bn)
15.2.5. Japan Drug And Gene Delivery Devices Market Size and Forecast, By Vector, 2023-2034 (Revenue USD Bn)
15.2.6. Japan Drug And Gene Delivery Devices Market Size and Forecast, By Route of Administration, 2023-2034 (Revenue USD Bn)
15.3. China Global Drug And Gene Delivery Devices Market
15.3.1. China Drug And Gene Delivery Devices Market Size and Forecast, 2023-2034 (Revenue USD Bn)
15.3.2. China Drug And Gene Delivery Devices Market Size and Forecast, By Product, 2023-2034 (Revenue USD Bn)
15.3.3. China Drug And Gene Delivery Devices Market Size and Forecast, By Commercialized Drugs, 2023-2034 (Revenue USD Bn)
15.3.4. China Drug And Gene Delivery Devices Market Size and Forecast, By Methods, 2023-2034 (Revenue USD Bn)
15.3.5. China Drug And Gene Delivery Devices Market Size and Forecast, By Vector, 2023-2034 (Revenue USD Bn)
15.3.6. China Drug And Gene Delivery Devices Market Size and Forecast, By Route of Administration, 2023-2034 (Revenue USD Bn)
15.4. India Global Drug And Gene Delivery Devices Market
15.4.1. India Drug And Gene Delivery Devices Market Size and Forecast, 2023-2034 (Revenue USD Bn)
15.4.2. India Drug And Gene Delivery Devices Market Size and Forecast, By Product, 2023-2034 (Revenue USD Bn)
15.4.3. India Drug And Gene Delivery Devices Market Size and Forecast, By Commercialized Drugs, 2023-2034 (Revenue USD Bn)
15.4.4. India Drug And Gene Delivery Devices Market Size and Forecast, By Methods, 2023-2034 (Revenue USD Bn)
15.4.5. India Drug And Gene Delivery Devices Market Size and Forecast, By Vector, 2023-2034 (Revenue USD Bn)
15.4.6. India Drug And Gene Delivery Devices Market Size and Forecast, By Route of Administration, 2023-2034 (Revenue USD Bn)
15.5. South Korea Global Drug And Gene Delivery Devices Market
15.5.1. South Korea Drug And Gene Delivery Devices Market Size and Forecast, 2023-2034 (Revenue USD Bn)
15.5.2. South Korea Drug And Gene Delivery Devices Market Size and Forecast, By Product, 2023-2034 (Revenue USD Bn)
15.5.3. South Korea Drug And Gene Delivery Devices Market Size and Forecast, By Commercialized Drugs, 2023-2034 (Revenue USD Bn)
15.5.4. South Korea Drug And Gene Delivery Devices Market Size and Forecast, By Methods, 2023-2034 (Revenue USD Bn)
15.5.5. South Korea Drug And Gene Delivery Devices Market Size and Forecast, By Vector, 2023-2034 (Revenue USD Bn)
15.5.6. South Korea Drug And Gene Delivery Devices Market Size and Forecast, By Route of Administration, 2023-2034 (Revenue USD Bn)
15.6. Australia Global Drug And Gene Delivery Devices Market
15.6.1. Australia Drug And Gene Delivery Devices Market Size and Forecast, 2023-2034 (Revenue USD Bn)
15.6.2. Australia Drug And Gene Delivery Devices Market Size and Forecast, By Product, 2023-2034 (Revenue USD Bn)
15.6.3. Australia Drug And Gene Delivery Devices Market Size and Forecast, By Commercialized Drugs, 2023-2034 (Revenue USD Bn)
15.6.4. Australia Drug And Gene Delivery Devices Market Size and Forecast, By Methods, 2023-2034 (Revenue USD Bn)
15.6.5. Australia Drug And Gene Delivery Devices Market Size and Forecast, By Vector, 2023-2034 (Revenue USD Bn)
15.6.6. Australia Drug And Gene Delivery Devices Market Size and Forecast, By Route of Administration, 2023-2034 (Revenue USD Bn)
15.7. Rest of Asia Pacific Global Drug And Gene Delivery Devices Market
15.7.1. Rest of Asia Pacific Drug And Gene Delivery Devices Market Size and Forecast, 2023-2034 (Revenue USD Bn)
15.7.2. Rest of Asia Pacific Drug And Gene Delivery Devices Market Size and Forecast, By Product, 2023-2034 (Revenue USD Bn)
15.7.3. Rest of Asia Pacific Drug And Gene Delivery Devices Market Size and Forecast, By Commercialized Drugs, 2023-2034 (Revenue USD Bn)
15.7.4. Rest of Asia Pacific Drug And Gene Delivery Devices Market Size and Forecast, By Methods, 2023-2034 (Revenue USD Bn)
15.7.5. Rest of Asia Pacific Drug And Gene Delivery Devices Market Size and Forecast, By Vector, 2023-2034 (Revenue USD Bn)
15.7.6. Rest of Asia Pacific Drug And Gene Delivery Devices Market Size and Forecast, By Route of Administration, 2023-2034 (Revenue USD Bn)
16. Latin America Global Drug And Gene Delivery Devices Market
16.1. Latin America Global Drug And Gene Delivery Devices Market
16.1.1. Latin America Drug And Gene Delivery Devices Market Size and Forecast, 2023-2034 (Revenue USD Bn)
16.1.2. Latin America Drug And Gene Delivery Devices Market Size and Forecast, By Country, 2023-2034 (Revenue USD Bn)
16.1.3. Latin America Drug And Gene Delivery Devices Market Size and Forecast, By Product, 2023-2034 (Revenue USD Bn)
16.1.4. Latin America Drug And Gene Delivery Devices Market Size and Forecast, By Commercialized Drugs, 2023-2034 (Revenue USD Bn)
16.1.5. Latin America Drug And Gene Delivery Devices Market Size and Forecast, By Methods, 2023-2034 (Revenue USD Bn)
16.1.6. Latin America Drug And Gene Delivery Devices Market Size and Forecast, By Vector, 2023-2034 (Revenue USD Bn)
16.1.7. Latin America Drug And Gene Delivery Devices Market Size and Forecast, By Route of Administration, 2023-2034 (Revenue USD Bn)
16.2. Brazil Global Drug And Gene Delivery Devices Market
16.2.1. Brazil Drug And Gene Delivery Devices Market Size and Forecast, 2023-2034 (Revenue USD Bn)
16.2.2. Brazil Drug And Gene Delivery Devices Market Size and Forecast, By Product, 2023-2034 (Revenue USD Bn)
16.2.3. Brazil Drug And Gene Delivery Devices Market Size and Forecast, By Commercialized Drugs, 2023-2034 (Revenue USD Bn)
16.2.4. Brazil Drug And Gene Delivery Devices Market Size and Forecast, By Methods, 2023-2034 (Revenue USD Bn)
16.2.5. Brazil Drug And Gene Delivery Devices Market Size and Forecast, By Vector, 2023-2034 (Revenue USD Bn)
16.2.6. Brazil Drug And Gene Delivery Devices Market Size and Forecast, By Route of Administration, 2023-2034 (Revenue USD Bn)
16.3. Mexico Global Drug And Gene Delivery Devices Market
16.3.1. Mexico Drug And Gene Delivery Devices Market Size and Forecast, 2023-2034 (Revenue USD Bn)
16.3.2. Mexico Drug And Gene Delivery Devices Market Size and Forecast, By Product, 2023-2034 (Revenue USD Bn)
16.3.3. Mexico Drug And Gene Delivery Devices Market Size and Forecast, By Commercialized Drugs, 2023-2034 (Revenue USD Bn)
16.3.4. Mexico Drug And Gene Delivery Devices Market Size and Forecast, By Methods, 2023-2034 (Revenue USD Bn)
16.3.5. Mexico Drug And Gene Delivery Devices Market Size and Forecast, By Vector, 2023-2034 (Revenue USD Bn)
16.3.6. Mexico Drug And Gene Delivery Devices Market Size and Forecast, By Route of Administration, 2023-2034 (Revenue USD Bn)
16.4. Argentina Global Drug And Gene Delivery Devices Market
16.4.1. Argentina Drug And Gene Delivery Devices Market Size and Forecast, 2023-2034 (Revenue USD Bn)
16.4.2. Argentina Drug And Gene Delivery Devices Market Size and Forecast, By Product, 2023-2034 (Revenue USD Bn)
16.4.3. Argentina Drug And Gene Delivery Devices Market Size and Forecast, By Commercialized Drugs, 2023-2034 (Revenue USD Bn)
16.4.4. Argentina Drug And Gene Delivery Devices Market Size and Forecast, By Methods, 2023-2034 (Revenue USD Bn)
16.4.5. Argentina Drug And Gene Delivery Devices Market Size and Forecast, By Vector, 2023-2034 (Revenue USD Bn)
16.4.6. Argentina Drug And Gene Delivery Devices Market Size and Forecast, By Route of Administration, 2023-2034 (Revenue USD Bn)
16.5. Rest of Latin America Global Drug And Gene Delivery Devices Market
16.5.1. Rest of Latin America Drug And Gene Delivery Devices Market Size and Forecast, 2023-2034 (Revenue USD Bn)
16.5.2. Rest of Latin America Drug And Gene Delivery Devices Market Size and Forecast, By Product, 2023-2034 (Revenue USD Bn)
16.5.3. Rest of Latin America Drug And Gene Delivery Devices Market Size and Forecast, By Commercialized Drugs, 2023-2034 (Revenue USD Bn)
16.5.4. Rest of Latin America Drug And Gene Delivery Devices Market Size and Forecast, By Methods, 2023-2034 (Revenue USD Bn)
16.5.5. Rest of Latin America Drug And Gene Delivery Devices Market Size and Forecast, By Vector, 2023-2034 (Revenue USD Bn)
16.5.6. Rest of Latin America Drug And Gene Delivery Devices Market Size and Forecast, By Route of Administration, 2023-2034 (Revenue USD Bn)
17. MEA Global Drug And Gene Delivery Devices Market
17.1. MEA Global Drug And Gene Delivery Devices Market
17.1.1. MEA Drug And Gene Delivery Devices Market Size and Forecast, 2023-2034 (Revenue USD Bn)
17.1.2. MEA Drug And Gene Delivery Devices Market Size and Forecast, By Country, 2023-2034 (Revenue USD Bn)
17.1.3. MEA Drug And Gene Delivery Devices Market Size and Forecast, By Product, 2023-2034 (Revenue USD Bn)
17.1.4. MEA Drug And Gene Delivery Devices Market Size and Forecast, By Commercialized Drugs, 2023-2034 (Revenue USD Bn)
17.1.5. MEA Drug And Gene Delivery Devices Market Size and Forecast, By Methods, 2023-2034 (Revenue USD Bn)
17.1.6. MEA Drug And Gene Delivery Devices Market Size and Forecast, By Vector, 2023-2034 (Revenue USD Bn)
17.1.7. MEA Drug And Gene Delivery Devices Market Size and Forecast, By Route of Administration, 2023-2034 (Revenue USD Bn)
17.2. GCC Global Drug And Gene Delivery Devices Market
17.2.1. GCC Drug And Gene Delivery Devices Market Size and Forecast, 2023-2034 (Revenue USD Bn)
17.2.2. GCC Drug And Gene Delivery Devices Market Size and Forecast, By Product, 2023-2034 (Revenue USD Bn)
17.2.3. GCC Drug And Gene Delivery Devices Market Size and Forecast, By Commercialized Drugs, 2023-2034 (Revenue USD Bn)
17.2.4. GCC Drug And Gene Delivery Devices Market Size and Forecast, By Methods, 2023-2034 (Revenue USD Bn)
17.2.5. GCC Drug And Gene Delivery Devices Market Size and Forecast, By Vector, 2023-2034 (Revenue USD Bn)
17.2.6. GCC Drug And Gene Delivery Devices Market Size and Forecast, By Route of Administration, 2023-2034 (Revenue USD Bn)
17.3. South Africa Global Drug And Gene Delivery Devices Market
17.3.1. South Africa Drug And Gene Delivery Devices Market Size and Forecast, 2023-2034 (Revenue USD Bn)
17.3.2. South Africa Drug And Gene Delivery Devices Market Size and Forecast, By Product, 2023-2034 (Revenue USD Bn)
17.3.3. South Africa Drug And Gene Delivery Devices Market Size and Forecast, By Commercialized Drugs, 2023-2034 (Revenue USD Bn)
17.3.4. South Africa Drug And Gene Delivery Devices Market Size and Forecast, By Methods, 2023-2034 (Revenue USD Bn)
17.3.5. South Africa Drug And Gene Delivery Devices Market Size and Forecast, By Vector, 2023-2034 (Revenue USD Bn)
17.3.6. South Africa Drug And Gene Delivery Devices Market Size and Forecast, By Route of Administration, 2023-2034 (Revenue USD Bn)
17.4. Rest of MEA Global Drug And Gene Delivery Devices Market
17.4.1. Rest of MEA Drug And Gene Delivery Devices Market Size and Forecast, 2023-2034 (Revenue USD Bn)
17.4.2. Rest of MEA Drug And Gene Delivery Devices Market Size and Forecast, By Product, 2023-2034 (Revenue USD Bn)
17.4.3. Rest of MEA Drug And Gene Delivery Devices Market Size and Forecast, By Commercialized Drugs, 2023-2034 (Revenue USD Bn)
17.4.4. Rest of MEA Drug And Gene Delivery Devices Market Size and Forecast, By Methods, 2023-2034 (Revenue USD Bn)
17.4.5. Rest of MEA Drug And Gene Delivery Devices Market Size and Forecast, By Vector, 2023-2034 (Revenue USD Bn)
17.4.6. Rest of MEA Drug And Gene Delivery Devices Market Size and Forecast, By Route of Administration, 2023-2034 (Revenue USD Bn)
18. Competitor Analysis
18.1. Company Market Share Analysis, 2023
18.2. Major Recent Developments
19. Company Profiles
19.1. Dendreon Pharmaceuticals
19.2. Pfizer, Inc.
19.3. Becton Dickinson and Company
19.4. Novartis AG
19.5. Bluebird bio, Inc.
19.6. Kolon Tissue Gene
19.7. Vericel Corporation
19.8. Renova Therapeutics
19.9. Orchard Therapeutics plc, Inc.
19.10. Amgen, Inc.
19.11. Kite Pharma, Inc.
19.12. Helixmith Co.
19.13. Bausch & Lomb Incorporated, Ltd (ViroMed Co., Ltd)
19.14. Spark Therapeutics, Inc.
19.15. uniQure N.V.
19.16. Castle Creek Biosciences, Inc (Fibrocell Technologies, Inc.)
19.17. Human Stem Cell Institute
19.18. Other Prominent Players