Rising approvals of advanced AI-based intracranial aneurysm devices is predicted to boost the market growth during the forecast period. A cerebral or intracranial aneurysm is an unusual bulging or dilation of an artery in the brain that occurs due to a weakening of the inner muscular layer (intima) of the blood vessel wall. This causes the vessel to develop a "blister-like" bulge that can become thin and burst unexpectedly, resulting in bleeding into the space around the brain, known as a subarachnoid hemorrhage (SAH). Aneurysms that are larger than one inch are classified as giant aneurysms, which present a particularly high risk and are challenging to treat. The precise mechanism underlying the development, growth, and rupture of cerebral aneurysms remains unknown. Treatment options for cerebral aneurysms encompass medical therapy, surgical clipping, endovascular therapy, and coiling, with or without additional devices. Consequently, market players are concentrating on the development of advanced AI-based devices. For instance, in February 2022, Viz.ai, a global leader in utilizing artificial intelligence (AI) for care coordination, has revealed that it obtained FDA 510(k) clearance for Viz ANEURYSM. This novel algorithm employs AI to identify potential cerebral aneurysms, allowing healthcare systems to guarantee that, once identified, patients are appropriately managed, and the workflow for aneurysm cases is standardized across the entire health system.
By type, endovascular coiling was the highest revenue-grossing segment in the global intracranial aneurysm market in 2023 owing to the rising advancements in products developed by key market players for the treatment of neurological disorders, and growing research & development activities. For instance, in January 2023, In-vivo study using Fluidx Medical Technology, Inc.'s IMPASS Embolic Device has shown promise, particularly when it comes to middle meningeal artery (MMA) embolizations. These procedures can be employed to treat chronic subdural hematomas (CSDH) located on the surface of the brain. Additionally, flow diverters is predicted to grow at fastest CAGR during the forecast period owing to the surge in product approvals, increasing demand for minimally invasive procedures, rising partnerships & collaborations within market players.
By end-user, hospital was the highest revenue-grossing segment in the global intracranial aneurysm market in 2023 owing to the growing utilization of highly advanced technology in operating rooms & intensive care units, increasing number of hospitals, surge in government funding for the hospitals, rising adoption of technologically advanced devices in hospitals. For instance, in September 2022, Siemens Healthineers' CorPath GRX Neurovascular System successfully completed 94% of its robotic-assisted endovascular procedures without the surgeon needing to switch to a manual approach during the operation. Additionally, clinics is predicted to grow at fastest CAGR during the forecast period owing to the improved healthcare facilities, the accessibility of state-of-the-art equipment, and rising preference by patients.
North America region is anticipated for the highest revenue share during the forecast period owing to the rising prevalence of intracranial aneurysms, hypertension, & stroke, existence of well-established healthcare facilities, increasing geriatric population, growing demand for minimally invasive procedures, surge in number of initiatives by various organizations, and rising focus on the development of innovative therapies, and increasing regulatory approvals. For instance, in September 2022, Vesalio has revealed that it has obtained FDA HDE (Humanitarian Device Exemption) approval for the commercialization of NeVa VS. This device is designed for use as an adjunct treatment for symptomatic cerebral vasospasm (CV) following aneurysmal subarachnoid hemorrhage (aSAH), which is a significant contributor to mortality and disability in this patient group. Additionally, Asia Pacific region is predicted to grow at fastest CAGR during the forecast period owing to the increasing prevalence of neurovascular illnesses, surge in government efforts, growing investment by manufacturers for research & development activities, rising improvement in the quality of diagnosis, and increasing launch of advanced devices. For instance, in June 2022, India Medtronic has introduced the fourth-generation flow diverter, Pipeline Vantage with Shield Technology, which has received CE marking. This device is used in the endovascular treatment of brain aneurysms.
Segmentation: Intracranial Aneurysm Market Report 2023 - 2034
Intracranial Aneurysm Market Analysis & Forecast by Type 2023 - 2034 (Revenue USD Bn)
- Flow Diverters
- Endovascular Coiling
- Surgical Clipping
- Others
Intracranial Aneurysm Market Analysis & Forecast by End-user 2023 - 2034 (Revenue USD Bn)
- Hospitals
- Clinics
- Others
Intracranial Aneurysm Market Analysis & Forecast by Region 2023 - 2034 (Revenue USD Bn)
- North America
- U.S.
- Canada
- Europe
- Germany
- France
- UK
- Spain
- Italy
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- Australia
- South Korea
- Rest of APAC
- Latin America
- Brazil
- Mexico
- Argentina
- Rest of LATAM
- Middle East & Africa
- South Africa
- GCC
- Rest of MEA
Table of Contents
Companies Mentioned
- Fluidx Medical Technology Inc.
- MicroPort Scientific Corporation
- Vesalio
- Medtronic
- Siemens Healthineers
- Integra LifeSciences
- Viz.ai
- Johnson & Johnson Services Inc.
- Stryker
- MicroVention Inc.
- B. Braun Melsungen AG
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 200 |
| Published | January 2024 |
| Forecast Period | 2023 - 2034 |
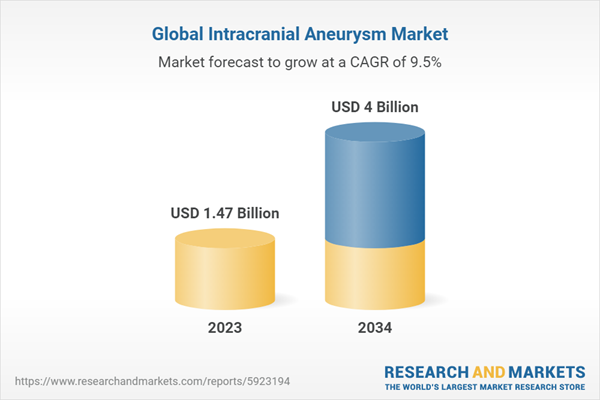
| Estimated Market Value ( USD | $ 1.47 Billion |
| Forecasted Market Value ( USD | $ 4 Billion |
| Compound Annual Growth Rate | 9.5% |
| Regions Covered | Global |
| No. of Companies Mentioned | 11 |