A peanut allergy is an adverse reaction to peanuts, typically arising when the body incorrectly perceives peanuts as harmful elements. Peanuts are recognized as a common source of severe allergic responses, and even a minimal quantity, such as one milligram, can induce a reaction. Consequently, prominent industry players are directing their efforts toward creating innovative treatment alternatives. Rising regulatory approvals for products treating peanut allergies is predicted to boost the market growth during the forecast period. For instance, in December 2023, Genentech, part of the Roche Group, revealed that the U.S. Food and Drug Administration (FDA) has given priority status to the company's supplemental Biologics License Application (sBLA) for Xolair (omalizumab). This application seeks approval for diminishing allergic reactions, including anaphylaxis, associated with accidental exposure to one or more foods in individuals aged 1 year and older with food allergies.
By drug class, epinephrine was the highest revenue-grossing segment in the global peanut allergy treatment market in 2023 owing to the heightened need for peanut allergy treatment is driven by the increasing occurrence of allergies, more stringent regulations on allergen labeling, and heightened awareness of the risks of anaphylaxis. There is an elevated emphasis on advancements in the design of auto-injectors, improved availability of epinephrine, and a commitment to ensuring prompt and efficient treatment for individuals with peanut allergies. For instance, in October 2022, The New Drug Application (NDA) for neffy, submitted by ARS Pharmaceuticals, Inc., has been acknowledged by the U.S. FDA. Neffy is designed for the immediate management of type I allergic reactions, including anaphylaxis, in both adults and children weighing at least 30 kg (66 lbs.). The FDA had initially established a target action date for the Prescription Drug User Fee Act (PDUFA) review, anticipated to be in the middle of 2023. Additionally, immunotherapies is predicted to grow at the fastest CAGR during forecast period. Two noteworthy approaches for peanut allergy treatment include Oral Immunotherapy (OIT) and Sublingual Immunotherapy (SLIT). OIT entails systematic exposure to minimal, regulated doses of peanut protein, progressively enhancing tolerance. Similarly, SLIT follows a comparable principle but delivers treatment beneath the tongue.
By route of administration, injectable was the highest revenue-grossing segment in the global peanut allergy treatment market in 2023 owing to the increasing emphasis on advancing innovative treatments. Epinephrine remains the primary medication for addressing anaphylaxis, and the preferred method of administration is an Intramuscular (IM) injection in the lateral thigh. Although Intravenous (IV) delivery is a viable option, it is primarily limited to hospital settings due to the requirement for close monitoring. Supportive therapies, such as bronchodilators, corticosteroids, and antihistamines, can be utilized, but they do not directly target the underlying condition. The administration of IV fluids is crucial for preventing and managing tissue hypoperfusion. For instance, in May 2023, DBV Technologies has declared the publication of Phase 3 EPITOPE trial results, which investigated EPIT with Viaskin Peanut in children aged 1 to 3 years, in the New England Journal of Medicine. This publication highlights the potential of a novel treatment option for food allergies in young children, presenting optimism for their effective management. Additionally, oral route of administration is predicted to grow at the fastest CAGR during the forecast period. Involving the gradual intake of regulated quantities of peanut protein, Oral Immunotherapy (OIT) presents various advantages for individuals managing peanut allergies. It also acts as a safeguard, reducing the risks associated with accidental exposure, which can be particularly crucial in emergency situations. Furthermore, successful OIT can improve overall quality of life, allowing individuals to dine with less anxiety and potentially reintroduce peanuts into their diet, broadening their food choices.
By distribution channel, hospital pharmacy was the highest revenue-grossing segment in the global peanut allergy treatment market in 2023 owing to the rising incidence of peanut allergies, escalating demand for treatments, and stringent regulations on allergen labeling have emphasized the necessity for timely access to peanut allergy treatments in hospital settings. Hospital pharmacies play a crucial role as a key distribution channel in the market, ensuring the availability and effective dispensing of medications and treatments vital for addressing allergies within a hospital setting. For instance, in June 2023, Alladapt Immunotherapeutics, Inc. has released the key findings from the Phase 1/2 Harmony study evaluating the efficacy and safety of ADP101 in the treatment of food allergies. The results demonstrate dose-dependent and clinically meaningful responses, along with a positive safety record, particularly in pediatric patients with either single or multiple food allergies. These encouraging results imply the potential of ADP101 as a safe and effective treatment option for children dealing with prevalent food allergies. Additionally, retail pharmacy is predicted to grow at the fastest CAGR during the forecast period owing to the importance of retail pharmacies is rooted in their accessibility, often strategically situated for patient convenience. This enables individuals to promptly acquire prescription medications and over-the-counter products, ultimately elevating the overall quality of care and enhancing the well-being of those managing peanut allergies. Retail pharmacies play a critical role in ensuring the widespread availability of these essential treatments for those requiring them.
North America region is anticipated for the highest revenue share during the forecast period owing to the increase in the number of adults & children grappling with peanut allergies, leading to a growing trend of collaborations among market players to introduce innovative treatments. For instance, in September 2023, Nestlé has sold its Palforzia business to Stallergenes Greer, a biopharmaceutical company specializing in allergy diagnosis and treatment. This decision is part of Nestlé's strategic move, which was preceded by their announcement to assess Palforzia. Under the agreement, Nestlé will receive milestone payments and continuous royalties. Additionally, Asia Pacific region is predicted to grow at fastest CAGR during the forecast period owing to the increasing incidence of food allergies specifically peanut allergies, a rising demand for effective therapies to manage and treat these allergies, a surge in the adoption of Oral Immunotherapy (OIT) & similar treatments, and increase in funding from key market players. For instance, in December 2022, Aravax has initiated its series B funding round and successfully secured a USD 20 million investment from well-known Australian healthcare entrepreneurs Tenmile and Brandon Capital. This funding will empower Aravax to initiate phase II clinical trials for PVX108, designed for individuals with peanut allergies.
Segmentation: Peanut Allergy Treatment Market Report 2023 - 2034
Peanut Allergy Treatment Market Analysis & Forecast by Drug Class 2023 - 2034 (Revenue USD Bn)
- Immunotherapies
- Epinephrine
- Antihistamines
- Others
Peanut Allergy Treatment Market Analysis & Forecast by Route of Administration 2023 - 2034 (Revenue USD Bn)
- Injectable
- Oral
- Others
Peanut Allergy Treatment Market Analysis & Forecast by Distribution Channel 2023 - 2034 (Revenue USD Bn)
- Retail Pharmacy
- Hospital Pharmacy
- Others
Peanut Allergy Treatment Market Analysis & Forecast by Region 2023 - 2034 (Revenue USD Bn)
- North America
- U.S.
- Canada
- Europe
- Germany
- France
- UK
- Spain
- Italy
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- Australia
- South Korea
- Rest of APAC
- Latin America
- Brazil
- Mexico
- Argentina
- Rest of LATAM
- Middle East & Africa
- South Africa
- GCC
- Rest of MEA
Table of Contents
Companies Mentioned
- FoRx Therapeutics
- Luciole Pharmaceuticals
- ClovisOncology Inc.
- Artios Pharma Limited
- AstraZeneca Plc
- Johnson & Johnson
- Onxeo
- GlaxoSmithKline plc.
- AbbVie
- Breakpoint Therapeutics
- Merck KGaA
- Pfizer Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 200 |
| Published | January 2024 |
| Forecast Period | 2023 - 2034 |
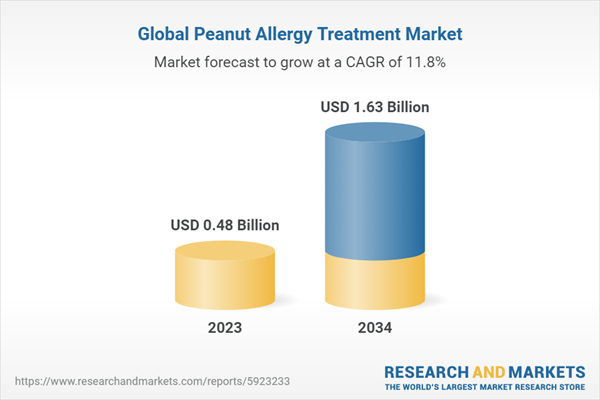
| Estimated Market Value ( USD | $ 0.48 Billion |
| Forecasted Market Value ( USD | $ 1.63 Billion |
| Compound Annual Growth Rate | 11.8% |
| Regions Covered | Global |
| No. of Companies Mentioned | 12 |