1. Research Methodology
1.1. Study Objectives
1.2. Study Scope
1.3. Research Assumptions
1.4. Research Framework
2. Introduction
2.1. Market Definition
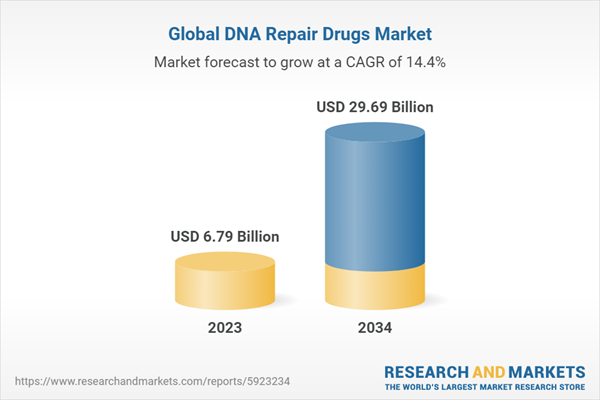
2.2. Global DNA Repair Drugs Market Overview
4. Market Environment Analysis
4.1. Porter’s 5 Forces Analysis
4.2. PESTEL Analysis
4.3. SWOT Analysis
5. Market Dynamics
5.1. Drivers Analysis
5.2. Restraints Analysis
5.3. Opportunities Analysis
5.4. Threats Analysis
5.5. Trend Analysis
7. DNA Repair Drugs Market: Drug Type Estimates & Trend Analysis
7.1. Drug Type Segment Opportunity Analysis
7.2. Niraparib
7.2.1. Niraparib Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
7.3. Olaparib
7.3.1. Olaparib Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
7.4. PARP Inhibitors
7.4.1. PARP Inhibitors Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
7.5. Talazoparib
7.5.1. Talazoparib Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
7.6. Rucaparib
7.6.1. Rucaparib Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
7.7. Others
7.7.1. Others Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
8. DNA Repair Drugs Market: Dosage Form Estimates & Trend Analysis
8.1. Dosage Form Segment Opportunity Analysis
8.2. Capsules
8.2.1. Capsules Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
8.3. Injectables
8.3.1. Injectables Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
8.4. Tablets
8.4.1. Tablets Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
9. DNA Repair Drugs Market: Application Estimates & Trend Analysis
9.1. Application Segment Opportunity Analysis
9.2. Peritoneal Cancer
9.2.1. Peritoneal Cancer Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
9.3. Ovarian Cancer
9.3.1. Ovarian Cancer Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
9.4. Breast Cancer
9.4.1. Breast Cancer Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
9.5. Fallopian Tube Cancer
9.5.1. Fallopian Tube Cancer Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
9.6. Others
9.6.1. Others Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
10. DNA Repair Drugs Market: Distribution Channel Estimates & Trend Analysis
10.1. Distribution Channel Segment Opportunity Analysis
10.2. Retail Pharmacies
10.2.1. Retail Pharmacies Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
10.3. Hospital Pharmacies
10.3.1. Hospital Pharmacies Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
10.4. Others
10.4.1. Others Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
11. Regional Market Analysis
11.1. Regional Market Opportunity Analysis
12. North America DNA Repair Drugs Market
12.1. North America DNA Repair Drugs Market
12.1.1. North America DNA Repair Drugs Market Size and Forecast, 2023-2034 (Revenue USD Bn)
12.1.2. North America DNA Repair Drugs Market Size and Forecast, By Country, 2023-2034 (Revenue USD Bn)
12.1.3. North America DNA Repair Drugs Market Size and Forecast, By Drug Type, 2023-2034 (Revenue USD Bn)
12.1.4. North America DNA Repair Drugs Market Size and Forecast, By Dosage Form, 2023-2034 (Revenue USD Bn)
12.1.5. North America DNA Repair Drugs Market Size and Forecast, By Application, 2023-2034 (Revenue USD Bn)
12.1.6. North America DNA Repair Drugs Market Size and Forecast, By Distribution Channel, 2023-2034 (Revenue USD Bn)
12.2. U.S. Global DNA Repair Drugs Market
12.2.1. U.S. DNA Repair Drugs Market Size and Forecast, 2023-2034 (Revenue USD Bn)
12.2.2. U.S. DNA Repair Drugs Market Size and Forecast, By Drug Type, 2023-2034 (Revenue USD Bn)
12.2.3. U.S. DNA Repair Drugs Market Size and Forecast, By Dosage Form, 2023-2034 (Revenue USD Bn)
12.2.4. U.S. DNA Repair Drugs Market Size and Forecast, By Application, 2023-2034 (Revenue USD Bn)
12.2.5. U.S. DNA Repair Drugs Market Size and Forecast, By Distribution Channel, 2023-2034 (Revenue USD Bn)
12.3. Canada Global DNA Repair Drugs Market
12.3.1. Canada DNA Repair Drugs Market Size and Forecast, 2023-2034 (Revenue USD Bn)
12.3.2. Canada DNA Repair Drugs Market Size and Forecast, By Drug Type, 2023-2034 (Revenue USD Bn)
12.3.3. Canada DNA Repair Drugs Market Size and Forecast, By Dosage Form, 2023-2034 (Revenue USD Bn)
12.3.4. Canada DNA Repair Drugs Market Size and Forecast, By Application, 2023-2034 (Revenue USD Bn)
12.3.5. Canada DNA Repair Drugs Market Size and Forecast, By Distribution Channel, 2023-2034 (Revenue USD Bn)
13. Europe Global DNA Repair Drugs Market
13.1. Europe Global DNA Repair Drugs Market
13.1.1. Europe DNA Repair Drugs Market Size and Forecast, 2023-2034 (Revenue USD Bn)
13.1.2. Europe DNA Repair Drugs Market Size and Forecast, By Country, 2023-2034 (Revenue USD Bn)
13.1.3. Europe DNA Repair Drugs Market Size and Forecast, By Drug Type, 2023-2034 (Revenue USD Bn)
13.1.4. Europe DNA Repair Drugs Market Size and Forecast, By Dosage Form, 2023-2034 (Revenue USD Bn)
13.1.5. Europe DNA Repair Drugs Market Size and Forecast, By Application, 2023-2034 (Revenue USD Bn)
13.1.6. Europe DNA Repair Drugs Market Size and Forecast, By Distribution Channel, 2023-2034 (Revenue USD Bn)
13.2. Germany Global DNA Repair Drugs Market
13.2.1. Germany DNA Repair Drugs Market Size and Forecast, 2023-2034 (Revenue USD Bn)
13.2.2. Germany DNA Repair Drugs Market Size and Forecast, By Drug Type, 2023-2034 (Revenue USD Bn)
13.2.3. Germany DNA Repair Drugs Market Size and Forecast, By Dosage Form, 2023-2034 (Revenue USD Bn)
13.2.4. Germany DNA Repair Drugs Market Size and Forecast, By Application, 2023-2034 (Revenue USD Bn)
13.2.5. Germany DNA Repair Drugs Market Size and Forecast, By Distribution Channel, 2023-2034 (Revenue USD Bn)
13.3. UK Global DNA Repair Drugs Market
13.3.1. UK DNA Repair Drugs Market Size and Forecast, 2023-2034 (Revenue USD Bn)
13.3.2. UK DNA Repair Drugs Market Size and Forecast, By Drug Type, 2023-2034 (Revenue USD Bn)
13.3.3. UK DNA Repair Drugs Market Size and Forecast, By Dosage Form, 2023-2034 (Revenue USD Bn)
13.3.4. UK DNA Repair Drugs Market Size and Forecast, By Application, 2023-2034 (Revenue USD Bn)
13.3.5. UK DNA Repair Drugs Market Size and Forecast, By Distribution Channel, 2023-2034 (Revenue USD Bn)
13.4. France Global DNA Repair Drugs Market
13.4.1. France DNA Repair Drugs Market Size and Forecast, 2023-2034 (Revenue USD Bn)
13.4.2. France DNA Repair Drugs Market Size and Forecast, By Drug Type, 2023-2034 (Revenue USD Bn)
13.4.3. France DNA Repair Drugs Market Size and Forecast, By Dosage Form, 2023-2034 (Revenue USD Bn)
13.4.4. France DNA Repair Drugs Market Size and Forecast, By Application, 2023-2034 (Revenue USD Bn)
13.4.5. France DNA Repair Drugs Market Size and Forecast, By Distribution Channel, 2023-2034 (Revenue USD Bn)
13.5. Spain Global DNA Repair Drugs Market
13.5.1. Spain DNA Repair Drugs Market Size and Forecast, 2023-2034 (Revenue USD Bn)
13.5.2. Spain DNA Repair Drugs Market Size and Forecast, By Drug Type, 2023-2034 (Revenue USD Bn)
13.5.3. Spain DNA Repair Drugs Market Size and Forecast, By Dosage Form, 2023-2034 (Revenue USD Bn)
13.5.4. Spain DNA Repair Drugs Market Size and Forecast, By Application, 2023-2034 (Revenue USD Bn)
13.5.5. Spain DNA Repair Drugs Market Size and Forecast, By Distribution Channel, 2023-2034 (Revenue USD Bn)
13.6. Italy Global DNA Repair Drugs Market
13.6.1. Italy DNA Repair Drugs Market Size and Forecast, 2023-2034 (Revenue USD Bn)
13.6.2. Italy DNA Repair Drugs Market Size and Forecast, By Drug Type, 2023-2034 (Revenue USD Bn)
13.6.3. Italy DNA Repair Drugs Market Size and Forecast, By Dosage Form, 2023-2034 (Revenue USD Bn)
13.6.4. Italy DNA Repair Drugs Market Size and Forecast, By Application, 2023-2034 (Revenue USD Bn)
13.6.5. Italy DNA Repair Drugs Market Size and Forecast, By Distribution Channel, 2023-2034 (Revenue USD Bn)
13.7. Rest of Europe Global DNA Repair Drugs Market
13.7.1. Rest of Europe DNA Repair Drugs Market Size and Forecast, 2023-2034 (Revenue USD Bn)
13.7.2. Rest of Europe DNA Repair Drugs Market Size and Forecast, By Drug Type, 2023-2034 (Revenue USD Bn)
13.7.3. Rest of Europe DNA Repair Drugs Market Size and Forecast, By Dosage Form, 2023-2034 (Revenue USD Bn)
13.7.4. Rest of Europe DNA Repair Drugs Market Size and Forecast, By Application, 2023-2034 (Revenue USD Bn)
13.7.5. Rest of Europe DNA Repair Drugs Market Size and Forecast, By Distribution Channel, 2023-2034 (Revenue USD Bn)
14. Asia Pacific Global DNA Repair Drugs Market
14.1. Asia Pacific Global DNA Repair Drugs Market
14.1.1. Asia Pacific DNA Repair Drugs Market Size and Forecast, 2023-2034 (Revenue USD Bn)
14.1.2. Asia Pacific DNA Repair Drugs Market Size and Forecast, By Country, 2023-2034 (Revenue USD Bn)
14.1.3. Asia Pacific DNA Repair Drugs Market Size and Forecast, By Drug Type, 2023-2034 (Revenue USD Bn)
14.1.4. Asia Pacific DNA Repair Drugs Market Size and Forecast, By Dosage Form, 2023-2034 (Revenue USD Bn)
14.1.5. Asia Pacific DNA Repair Drugs Market Size and Forecast, By Application, 2023-2034 (Revenue USD Bn)
14.1.6. Asia Pacific DNA Repair Drugs Market Size and Forecast, By Distribution Channel, 2023-2034 (Revenue USD Bn)
14.2. Japan Global DNA Repair Drugs Market
14.2.1. Japan DNA Repair Drugs Market Size and Forecast, 2023-2034 (Revenue USD Bn)
14.2.2. Japan DNA Repair Drugs Market Size and Forecast, By Drug Type, 2023-2034 (Revenue USD Bn)
14.2.3. Japan DNA Repair Drugs Market Size and Forecast, By Dosage Form, 2023-2034 (Revenue USD Bn)
14.2.4. Japan DNA Repair Drugs Market Size and Forecast, By Application, 2023-2034 (Revenue USD Bn)
14.2.5. Japan DNA Repair Drugs Market Size and Forecast, By Distribution Channel, 2023-2034 (Revenue USD Bn)
14.3. China Global DNA Repair Drugs Market
14.3.1. China DNA Repair Drugs Market Size and Forecast, 2023-2034 (Revenue USD Bn)
14.3.2. China DNA Repair Drugs Market Size and Forecast, By Drug Type, 2023-2034 (Revenue USD Bn)
14.3.3. China DNA Repair Drugs Market Size and Forecast, By Dosage Form, 2023-2034 (Revenue USD Bn)
14.3.4. China DNA Repair Drugs Market Size and Forecast, By Application, 2023-2034 (Revenue USD Bn)
14.3.5. China DNA Repair Drugs Market Size and Forecast, By Distribution Channel, 2023-2034 (Revenue USD Bn)
14.4. India Global DNA Repair Drugs Market
14.4.1. India DNA Repair Drugs Market Size and Forecast, 2023-2034 (Revenue USD Bn)
14.4.2. India DNA Repair Drugs Market Size and Forecast, By Drug Type, 2023-2034 (Revenue USD Bn)
14.4.3. India DNA Repair Drugs Market Size and Forecast, By Dosage Form, 2023-2034 (Revenue USD Bn)
14.4.4. India DNA Repair Drugs Market Size and Forecast, By Application, 2023-2034 (Revenue USD Bn)
14.4.5. India DNA Repair Drugs Market Size and Forecast, By Distribution Channel, 2023-2034 (Revenue USD Bn)
14.5. South Korea Global DNA Repair Drugs Market
14.5.1. South Korea DNA Repair Drugs Market Size and Forecast, 2023-2034 (Revenue USD Bn)
14.5.2. South Korea DNA Repair Drugs Market Size and Forecast, By Drug Type, 2023-2034 (Revenue USD Bn)
14.5.3. South Korea DNA Repair Drugs Market Size and Forecast, By Dosage Form, 2023-2034 (Revenue USD Bn)
14.5.4. South Korea DNA Repair Drugs Market Size and Forecast, By Application, 2023-2034 (Revenue USD Bn)
14.5.5. South Korea DNA Repair Drugs Market Size and Forecast, By Distribution Channel, 2023-2034 (Revenue USD Bn)
14.6. Australia Global DNA Repair Drugs Market
14.6.1. Australia DNA Repair Drugs Market Size and Forecast, 2023-2034 (Revenue USD Bn)
14.6.2. Australia DNA Repair Drugs Market Size and Forecast, By Drug Type, 2023-2034 (Revenue USD Bn)
14.6.3. Australia DNA Repair Drugs Market Size and Forecast, By Dosage Form, 2023-2034 (Revenue USD Bn)
14.6.4. Australia DNA Repair Drugs Market Size and Forecast, By Application, 2023-2034 (Revenue USD Bn)
14.6.5. Australia DNA Repair Drugs Market Size and Forecast, By Distribution Channel, 2023-2034 (Revenue USD Bn)
14.7. Rest of Asia Pacific Global DNA Repair Drugs Market
14.7.1. Rest of Asia Pacific DNA Repair Drugs Market Size and Forecast, 2023-2034 (Revenue USD Bn)
14.7.2. Rest of Asia Pacific DNA Repair Drugs Market Size and Forecast, By Drug Type, 2023-2034 (Revenue USD Bn)
14.7.3. Rest of Asia Pacific DNA Repair Drugs Market Size and Forecast, By Dosage Form, 2023-2034 (Revenue USD Bn)
14.7.4. Rest of Asia Pacific DNA Repair Drugs Market Size and Forecast, By Application, 2023-2034 (Revenue USD Bn)
14.7.5. Rest of Asia Pacific DNA Repair Drugs Market Size and Forecast, By Distribution Channel, 2023-2034 (Revenue USD Bn)
15. Latin America Global DNA Repair Drugs Market
15.1. Latin America Global DNA Repair Drugs Market
15.1.1. Latin America DNA Repair Drugs Market Size and Forecast, 2023-2034 (Revenue USD Bn)
15.1.2. Latin America DNA Repair Drugs Market Size and Forecast, By Country, 2023-2034 (Revenue USD Bn)
15.1.3. Latin America DNA Repair Drugs Market Size and Forecast, By Drug Type, 2023-2034 (Revenue USD Bn)
15.1.4. Latin America DNA Repair Drugs Market Size and Forecast, By Dosage Form, 2023-2034 (Revenue USD Bn)
15.1.5. Latin America DNA Repair Drugs Market Size and Forecast, By Application, 2023-2034 (Revenue USD Bn)
15.1.6. Latin America DNA Repair Drugs Market Size and Forecast, By Distribution Channel, 2023-2034 (Revenue USD Bn)
15.2. Brazil Global DNA Repair Drugs Market
15.2.1. Brazil DNA Repair Drugs Market Size and Forecast, 2023-2034 (Revenue USD Bn)
15.2.2. Brazil DNA Repair Drugs Market Size and Forecast, By Drug Type, 2023-2034 (Revenue USD Bn)
15.2.3. Brazil DNA Repair Drugs Market Size and Forecast, By Dosage Form, 2023-2034 (Revenue USD Bn)
15.2.4. Brazil DNA Repair Drugs Market Size and Forecast, By Application, 2023-2034 (Revenue USD Bn)
15.2.5. Brazil DNA Repair Drugs Market Size and Forecast, By Distribution Channel, 2023-2034 (Revenue USD Bn)
15.3. Mexico Global DNA Repair Drugs Market
15.3.1. Mexico DNA Repair Drugs Market Size and Forecast, 2023-2034 (Revenue USD Bn)
15.3.2. Mexico DNA Repair Drugs Market Size and Forecast, By Drug Type, 2023-2034 (Revenue USD Bn)
15.3.3. Mexico DNA Repair Drugs Market Size and Forecast, By Dosage Form, 2023-2034 (Revenue USD Bn)
15.3.4. Mexico DNA Repair Drugs Market Size and Forecast, By Application, 2023-2034 (Revenue USD Bn)
15.3.5. Mexico DNA Repair Drugs Market Size and Forecast, By Distribution Channel, 2023-2034 (Revenue USD Bn)
15.4. Argentina Global DNA Repair Drugs Market
15.4.1. Argentina DNA Repair Drugs Market Size and Forecast, 2023-2034 (Revenue USD Bn)
15.4.2. Argentina DNA Repair Drugs Market Size and Forecast, By Drug Type, 2023-2034 (Revenue USD Bn)
15.4.3. Argentina DNA Repair Drugs Market Size and Forecast, By Dosage Form, 2023-2034 (Revenue USD Bn)
15.4.4. Argentina DNA Repair Drugs Market Size and Forecast, By Application, 2023-2034 (Revenue USD Bn)
15.4.5. Argentina DNA Repair Drugs Market Size and Forecast, By Distribution Channel, 2023-2034 (Revenue USD Bn)
15.5. Rest of Latin America Global DNA Repair Drugs Market
15.5.1. Rest of Latin America DNA Repair Drugs Market Size and Forecast, 2023-2034 (Revenue USD Bn)
15.5.2. Rest of Latin America DNA Repair Drugs Market Size and Forecast, By Drug Type, 2023-2034 (Revenue USD Bn)
15.5.3. Rest of Latin America DNA Repair Drugs Market Size and Forecast, By Dosage Form, 2023-2034 (Revenue USD Bn)
15.5.4. Rest of Latin America DNA Repair Drugs Market Size and Forecast, By Application, 2023-2034 (Revenue USD Bn)
15.5.5. Rest of Latin America DNA Repair Drugs Market Size and Forecast, By Distribution Channel, 2023-2034 (Revenue USD Bn)
16. MEA Global DNA Repair Drugs Market
16.1. MEA Global DNA Repair Drugs Market
16.1.1. MEA DNA Repair Drugs Market Size and Forecast, 2023-2034 (Revenue USD Bn)
16.1.2. MEA DNA Repair Drugs Market Size and Forecast, By Country, 2023-2034 (Revenue USD Bn)
16.1.3. MEA DNA Repair Drugs Market Size and Forecast, By Drug Type, 2023-2034 (Revenue USD Bn)
16.1.4. MEA DNA Repair Drugs Market Size and Forecast, By Dosage Form, 2023-2034 (Revenue USD Bn)
16.1.5. MEA DNA Repair Drugs Market Size and Forecast, By Application, 2023-2034 (Revenue USD Bn)
16.1.6. MEA DNA Repair Drugs Market Size and Forecast, By Distribution Channel, 2023-2034 (Revenue USD Bn)
16.2. GCC Global DNA Repair Drugs Market
16.2.1. GCC DNA Repair Drugs Market Size and Forecast, 2023-2034 (Revenue USD Bn)
16.2.2. GCC DNA Repair Drugs Market Size and Forecast, By Drug Type, 2023-2034 (Revenue USD Bn)
16.2.3. GCC DNA Repair Drugs Market Size and Forecast, By Dosage Form, 2023-2034 (Revenue USD Bn)
16.2.4. GCC DNA Repair Drugs Market Size and Forecast, By Application, 2023-2034 (Revenue USD Bn)
16.2.5. GCC DNA Repair Drugs Market Size and Forecast, By Distribution Channel, 2023-2034 (Revenue USD Bn)
16.3. South Africa Global DNA Repair Drugs Market
16.3.1. South Africa DNA Repair Drugs Market Size and Forecast, 2023-2034 (Revenue USD Bn)
16.3.2. South Africa DNA Repair Drugs Market Size and Forecast, By Drug Type, 2023-2034 (Revenue USD Bn)
16.3.3. South Africa DNA Repair Drugs Market Size and Forecast, By Dosage Form, 2023-2034 (Revenue USD Bn)
16.3.4. South Africa DNA Repair Drugs Market Size and Forecast, By Application, 2023-2034 (Revenue USD Bn)
16.3.5. South Africa DNA Repair Drugs Market Size and Forecast, By Distribution Channel, 2023-2034 (Revenue USD Bn)
16.4. Rest of MEA Global DNA Repair Drugs Market
16.4.1. Rest of MEA DNA Repair Drugs Market Size and Forecast, 2023-2034 (Revenue USD Bn)
16.4.2. Rest of MEA DNA Repair Drugs Market Size and Forecast, By Drug Type, 2023-2034 (Revenue USD Bn)
16.4.3. Rest of MEA DNA Repair Drugs Market Size and Forecast, By Dosage Form, 2023-2034 (Revenue USD Bn)
16.4.4. Rest of MEA DNA Repair Drugs Market Size and Forecast, By Application, 2023-2034 (Revenue USD Bn)
16.4.5. Rest of MEA DNA Repair Drugs Market Size and Forecast, By Distribution Channel, 2023-2034 (Revenue USD Bn)
17. Competitor Analysis
17.1. Company Market Share Analysis, 2023
17.2. Major Recent Developments
18. Company Profiles
18.1. FoRx Therapeutics
18.2. Luciole Pharmaceuticals
18.3. ClovisOncology Inc.
18.4. Artios Pharma Limited
18.5. AstraZeneca Plc
18.6. Johnson & Johnson
18.7. Onxeo
18.8. GlaxoSmithKline plc.
18.9. AbbVie
18.10. Breakpoint Therapeutics
18.11. Merck KGaA
18.12. Pfizer Inc.
18.13. Other Prominent Players