Increasing Adoption and Outsourcing of Clinical Trials Fuels Europe clinical trials market.
Clinical trials help determine if a new form of treatment or prevention, such as a new drug, diet, or medical device, is safe and effective. The trials are mainly carried out during drug development. According to European Medicine Agency, in the European Union (EU), ~4,000 clinical trials are authorized annually, of which about 60% of clinical trials are associated with the pharmaceutical industry. An increasing number of clinical trials for developing different effective treatments due to the rising prevalence of chronic diseases is fueling the growth of the clinical trials market.
Further, clinical trials are increasingly becoming complex procedures, owing to which proper execution and overseeing of the operation occurring in research-based organizations has become crucial. To avoid errors due to improper execution, research-based organizations are outsourcing clinical trials to develop their products. Clinical research organizations (CROs) assist in successfully implementing clinical trials through the services offered using high-quality facilities and deep subject matter expertise. CROs have begun acting as a backbone of the clinical trial industry through their efficient and cost-effective operations benefitting trial sponsors. Thus, the development of cost-effective solutions and decreasing errors in CROs during the drug development process are driving the clinical trials market growth.
Europe Clinical Trials Market Overview
The Europe clinical trials market has been segmented into the UK, Germany, France, Italy, Spain, and the Rest of Europe. The rising number of clinical trial activities for new drug development and innovative product launches by the companies will likely enhance the market growth in Europe during the forecast period.Cancer, cardiovascular illness, nervous system disorders, and infectious diseases are the most frequently studied conditions in clinical trials in Germany. Clinical research benefits from Germany's well-established transport, communication, energy, and public services infrastructure, Moreover, there is a large patient pool and a high demand for quality healthcare in the country. Coordinating centers for clinical trials were established as part of a new funding program under the Federal Ministry of Education and Research to foster academic clinical research. Clinical trials in Germany are approved by the Federal Institute for Drugs and Medical Devices or the Paul-Ehrlich Institute, depending on the product to be investigated. Therefore, Germany's clinical trial approval processes are standardized, reliable, transparent, and approved for relatively short study start-up timelines. Further, top CROs in Germany provide several clinical trial services. Sofpromed manages clinical trials in Germany and other EU member states. It offers a full range of CRO services for pharmaceutical and biotechnology companies. Similarly, CONET GmbH, headquartered in Mannheim, Germany, offers clinical trial management services from small pilot studies to large multicenter international clinical trials for all phases (1-4), including pediatric clinical trials and medical device trials as per EU Directives.
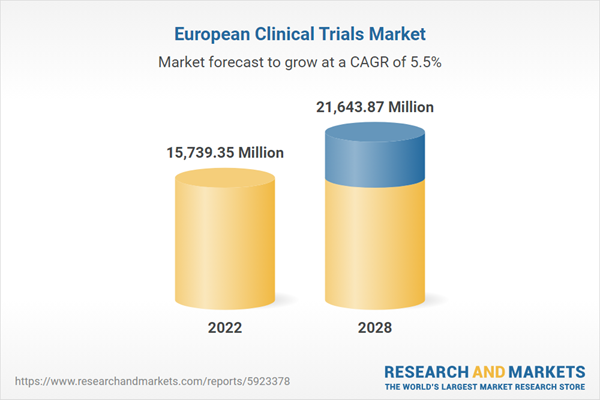
Europe clinical trials market Revenue and Forecast to 2028 (US$ Million)
Europe Clinical Trials Market Segmentation
The Europe clinical trials market is segmented into phase, study design, indication, and country.Based on phase, the Europe clinical trials market is segmented into phase I, phase II, phase III, and phase IV. The phase III segment registered the largest Europe clinical trials market share in 2022.
Based on study design, the Europe clinical trials market is segmented into interventional, observational, and expanded access. The interventional segment held the largest Europe clinical trials market share in 2022.
Based on indication, the Europe clinical trials market is segmented into autoimmune/inflammation, pain management, oncology, CNS condition, diabetes, obesity, cardiovascular, and others. The oncology segment held the largest Europe clinical trials market share in 2022.
Based on country, the Europe clinical trials market has been categorized into the UK, Germany, France, Italy, Spain, and Rest of Europe. Germany dominated the Europe clinical trials market in 2022.
Charles River Laboratories International Inc, ICON Plc, IQVIA Holdings Inc, IXICO Plc, Laboratory Corp of America Holdings, Parexel International Corp, SGS SA, Syneos Health Inc, Thermo Fisher Scientific Inc, and WuXi AppTec Co Ltd. are some of the leading companies operating in the Europe clinical trials market.
Reasons to Buy
- Save and reduce time carrying out entry-level research by identifying the growth, size, leading players, and segments in the Europe clinical trials market.
- Highlights key business priorities in order to assist companies to realign their business strategies
- The key findings and recommendations highlight crucial progressive industry trends in the Europe clinical trials market, thereby allowing players across the value chain to develop effective long-term strategies
- Develop/modify business expansion plans by using substantial growth offering developed and emerging markets
- Scrutinize in-depth Europe market trends and outlook coupled with the factors driving the clinical trials market, as well as those hindering it
- Enhance the decision-making process by understanding the strategies that underpin commercial interest with respect to client products, segmentation, pricing, and distribution
Table of Contents
Companies Mentioned
- Charles River Laboratories International Inc
- ICON Plc
- IQVIA Holdings Inc
- IXICO Plc
- Laboratory Corp of America Holdings
- Parexel International Corp
- SGS SA
- Syneos Health Inc
- Thermo Fisher Scientific Inc
- WuXi AppTec Co Ltd
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 148 |
| Published | October 2023 |
| Forecast Period | 2022 - 2028 |
| Estimated Market Value in 2022 | 15739.35 Million |
| Forecasted Market Value by 2028 | 21643.87 Million |
| Compound Annual Growth Rate | 5.5% |
| Regions Covered | Europe |
| No. of Companies Mentioned | 10 |