A spinal cord stimulation device is an implanted device that sends low levels of electricity directly into the spinal cord to relieve pain. A spinal cord stimulator is mostly used after non-surgical pain treatment options that have failed to provide sufficient relief. Additionally, a spinal cord stimulator can improve the overall quality of life and sleep and reduce the need for pain medicines. It is typically used along with other pain management treatments.
Availability of Innovative Treatments in the European Union
According to a report published by the European Commission, SCI is responsible for causing motor impairment with sensation loss, chronic pain, and other locomotor dysfunction. Therefore, in the European Union (EU), several rehabilitation programs are being conducted for patients living with SCIs. The SPINAL CORD REPAIR Project (SCRP) is one such example that is a multidisciplinary collaboration among EU scientists. The SCRP is responsible for restoring physical movements such as motor function or movement after SCIs. Under this program, an experimental setup - MotoRater - was developed and trademarked for the standardized testing of locomotion movements among rodents as a part of clinical testing. These rodents were utilized to identify and characterize the cellular properties of excitatory central pattern generator (CPG) neurons such as EphA4, which are responsible for locomotion. CPG is a neural circuit in the spinal cord that stimulates rhythmic movements from the interplay between CPG neurons and their synaptic interactions. The clinical research results were presented through meetings and congresses; patents were filed and published. Therefore, the ongoing innovations in the treatments of spinal cord disorders in the EU help improve the mobility and quality of life for millions suffering from SCI.Several biopharma companies are developing innovative products to treat SCIs. BioArctic, a Swedish biopharma company, is dedicated to developing treatments for central nervous system (CNS) disorders. For instance, in February 2019, BioArctic developed"SC0806 a biodegradable medical device that is surgically implanted into an injured spinal cord. Upon the implantation of"SCO806 the device guides nerve fibers across the injured area in the spinal cord. Such innovative product launches by competitive players to treat SCI fuel the growth of spinal cord stimulation devices for the chronic pain market in Europe and the Middle East.
Market Trend
Regenerative and Innovative Implant-Based Therapeutic Approaches
The Comunidad de Madrid claims to be the first in the EU to provide treatments for SCIs using cell and regenerative therapy. Regenerative medicine includes the extraction of mesenchymal stem cells from patients suffering SCIs, treating the extracted cells in a cell production room, and then injecting the regenerated cells at the exact site of SCI or into the cerebrospinal fluid (CSF). Thus, this technique serves as a personalized treatment for SCIs.The European Commission published a report in September 2022 revealing that scientists are developing new ways to treat SCI using graphene-based implants and virtual reality (VR) gaming to help improve stroke recovery. Scientists are trying these new approaches to reverse nerve damage, while some researchers are attempting to reshape the architecture of the spinal cord in situ by using engineered materials such as graphene. Graphene is used to create a 3D structure that would skillfully mimic the morphology of the spinal cord. Therefore, the use of such innovative engineered materials and implants successfully exhibits the treatment potential in patients suffering from SCIs. The regenerative and innovative implant-based therapeutic approaches are emerging as new trends in Europe and the Middle East spinal cord stimulation devices for the chronic pain market.
The “Europe and Middle East Spinal Cord Stimulation Devices for Chronic Pain Market” is segmented on the basis of product type, application, end-user, and region. Based on product type, the spinal cord stimulation devices for chronic pain market is bifurcated into rechargeable and non-rechargeable. The rechargeable segment held the largest market share in 2022, and the same segment is expected to record a significant CAGR during 2022-2030. Based on application, the spinal cord stimulation devices for the chronic pain market are segmented as failed back syndrome, complex regional pain syndrome, degenerative disk disease, and others. The complex regional pain syndrome segment held the largest market share in 2022, and the same segment is anticipated to register the highest CAGR for the forecast period 2020-2030. In terms of end users, the spinal cord stimulation devices for the chronic pain market are categorized into hospitals, ambulatory surgery centers, and others. The hospital segment held a larger share of the market in 2022 and is anticipated to register a higher CAGR during 2022-2030.
In September 2023, Resonant Link and Cirtec Medical Corp partnered to power the future of implantable medical devices. By combining Resonant Link’s next-generation wireless power with Cirtec Medical’s expert product design, development, and manufacturing services, this partnership will offer medical device makers the best technology, fully integrated components, and faster time to market.
Reasons to Buy
- Save and reduce time carrying out entry-level research by identifying the growth, size, leading players and segments in the Europe and Middle East spinal cord stimulation devices for chronic pain market.
- Highlights key business priorities in order to assist companies to realign their business strategies.
- The key findings and recommendations highlight crucial progressive industry trends in the global Europe and Middle East spinal cord stimulation devices for chronic pain market, thereby allowing players across the value chain to develop effective long-term strategies.
- Develop/modify business expansion plans by using substantial growth offering developed and emerging markets.
- Scrutinize in-depth global market trends and outlook coupled with the factors driving the market, as well as those hindering it.
- Enhance the decision-making process by understanding the strategies that underpin security interest with respect to client products, segmentation, pricing and distribution.
Table of Contents
Companies Mentioned
- Boston Scientific Corp
- Abbott Laboratories
- Medtronic Plc
- Curonix LLC
- Biotronik SE & Co KG
- Nevro Corp
- Nalu Medical Inc
- Cirtec Medical Corp
- Synapse Biomedical Inc
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 204 |
| Published | November 2023 |
| Forecast Period | 2022 - 2030 |
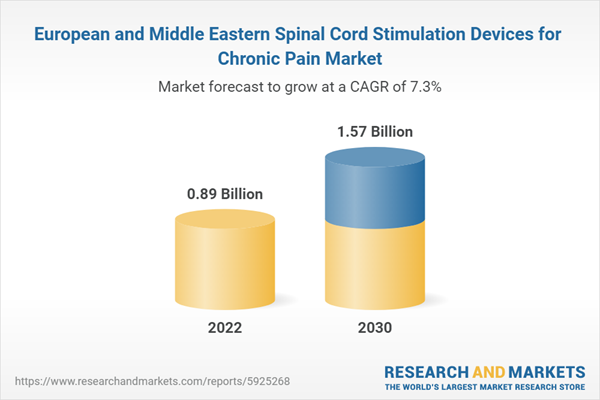
| Estimated Market Value in 2022 | 0.89 Billion |
| Forecasted Market Value by 2030 | 1.57 Billion |
| Compound Annual Growth Rate | 7.3% |
| Regions Covered | Europe, Middle East |
| No. of Companies Mentioned | 9 |