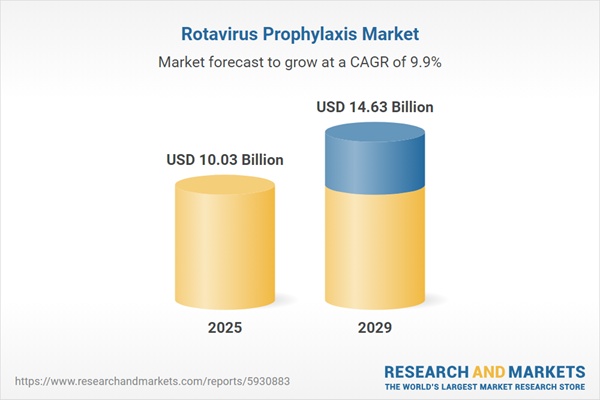
The rotavirus prophylaxis market size has grown rapidly in recent years. It will grow from $9.05 billion in 2024 to $10.03 billion in 2025 at a compound annual growth rate (CAGR) of 10.8%. The growth in the historic period can be attributed to vaccine access and equity, emerging rotavirus strains, r&d investments, public health preparedness.
The rotavirus prophylaxis market size is expected to see strong growth in the next few years. It will grow to $14.63 billion in 2029 at a compound annual growth rate (CAGR) of 9.9%. The growth in the forecast period can be attributed to gastrointestinal disease burden, global expansion, vaccination programs, vaccine confidence. Major trends in the forecast period include next-generation vaccines, combination vaccines, vaccine delivery innovation, global immunization collaboration.
The growth of the rotavirus prophylaxis market is anticipated to be powered by the rising number of vaccine courses. A vaccine course, which consists of specific doses of a vaccine designed to confer protection against a particular disease, plays a pivotal role in the battle against rotavirus infection. The vaccine course dosage acts as a catalyst for the immunological response, significantly enhancing immunity against rotavirus and reducing the risk of illness. This leads to an overall increase in vaccine efficacy. As an illustration, a report by the United Nations International Children's Emergency Fund (UNICEF) published in January 2022 revealed that approximately 5.71 million courses of RV1-5 frozen rotavirus vaccine were manufactured in 2022, with a projected surge to 9.31 million courses by 2028. Furthermore, 5.29 million courses of RV5-2 lyophilized rotavirus vaccines were produced in 2022, with an expected rise to 6.13 million courses by 2028. Consequently, the growing availability of vaccines will be a driving force behind the rotavirus prophylaxis market.
The rotavirus prophylaxis market is expected to experience significant growth fueled by institutional funding. Institutional funding entails the allocation of financial, human, or material resources to support various programs, needs, or projects. The escalating incidence of rotavirus cases has attracted funding from government bodies and private organizations. This financial support encompasses both monetary and technical assistance, spanning different stages of rotavirus vaccine and oral medication development. To exemplify, in April 2022, Indiana University, a public research university based in the United States, secured a $1.2 million grant from the venture capital firm GIVAX Inc. The grant aims to facilitate the development of innovative technology for a combined oral rotavirus-norovirus vaccine for infants. This groundbreaking technology expands the scope of the existing rotavirus vaccine to include protection against norovirus, a highly contagious virus responsible for severe vomiting and diarrhea in young children. Therefore, the increased availability of funding sources is poised to propel the rotavirus prophylaxis market.
Prominent companies operating in the rotavirus prophylaxis market are actively engaged in vaccine development to meet the evolving demands of consumers. The process of vaccine approvals involves a rigorous and multi-stage development and testing procedure. These approvals are essential in ensuring the safety and effectiveness of vaccines for preventing this viral disease, especially in infants and young children. For instance, in November 2022, GlaxoSmithKline PLC, a U.K.-based pharmaceutical and biotechnology company, announced the approval by the Food and Drug Administration (FDA) of an oral-dosing applicator-only presentation (liquid formulation) of ROTARIX (Rotavirus Vaccine, Live and Oral). This vaccine prevents rotavirus gastroenteritis in infants, caused by G1 and non-G1 types (G3, G4, and G9). The novel oral-dosing applicator-only presentation is entirely liquid and should not be reconstituted before use. Both ROTARIX lyophilized and liquid formulations are administered orally in a two-dose series, containing the same live, human-attenuated rotavirus strain and manufactured using similar methods.
In August 2022, Aspen Pharmacare Holdings Limited (APN), a South Africa-based pharmaceutical company, entered into a strategic collaboration with Serum Institute of India Pvt. Ltd. The partnership with the Serum Institute of India (SII) aimed to manufacture and distribute four Aspen-branded vaccines in Africa. This collaboration sought to bolster Africa's health manufacturing capacity and provide affordable vaccine access to the African population. Serum Institute of India Pvt. Ltd. is a vaccine manufacturer based in India, including vaccines for rotavirus.
Major companies operating in the rotavirus prophylaxis market are Pfizer Inc., Johnson & Johnson, Merck & Co., Novartis AG, Sanofi SA, Astra Zeneca Pharmaceuticals LP, GlaxoSmithKline PLC, Takeda Pharmaceutical Company Limited, Sinovac Biotech Ltd., Astellas Pharma Inc., CSL Limited, Bharat Biotech International Limited, Daiichi Sankyo Co. Ltd., Lupin Limited, Mitsubishi Tanabe Pharma Corporation, Serum Institute of India Pvt. Ltd., Zydus Lifescience Ltd., Emergent BioSolutions Inc., Hualan Biological Engineering Inc., Biocad Biopharmaceutical Co., Bharat Serums and Vaccines Limited, Biological E Limited, PT Bio Farma, Panacea Biotec Ltd., Shanghai Institute of Biological Products (SIBP).
North America was the largest region in the rotavirus prophylaxis market in 2024. The regions covered in rotavirus prophylaxis report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East and Africa. The countries covered in the rotavirus prophylaxis market report are Australia, Brazil, China, France, Germany, India, Indonesia, Japan, Russia, South Korea, UK, USA, Canada, Italy, Spain.
The rotavirus prophylaxis market consists of revenues earned by entities by providing services such as vaccination, clinical management, awareness programs and diagnosis. Values in this market are ‘factory gate’ values, that is, the value of goods sold by the manufacturers or creators of the goods, whether to other entities (including downstream manufacturers, wholesalers, distributors and retailers) or directly to end customers. The value of goods in this market includes related services sold by the creators of the goods.
Rotavirus prophylaxis involves the prevention of rotavirus infection through vaccination, aiming to shield infants and young children from this highly contagious illness that triggers inflammation in the stomach and bowels. The primary objective is to provide protection against the infection's severity.
The primary methods employed for rotavirus prophylaxis include the administration of vaccines such as Rotarix, Rotavac, Rotavin-M1, as well as the utilization of oral rehydration fluids, among other interventions. Rotavirus, a viral agent, is responsible for inducing severe stomach and intestinal disorders, often characterized by symptoms like fever, vomiting, and diarrhea. These interventions are deployed through various routes of administration, including oral and parenteral methods, and are readily accessible through outlets such as hospital pharmacies, retail pharmacies, online pharmacy platforms, and others. The intended beneficiaries of these preventive measures encompass hospitals, specialty clinics, home care services, and various other healthcare settings.
The rotavirus prophylaxis market research report is one of a series of new reports that provides rotavirus prophylaxis market statistics, including rotavirus prophylaxis industry global market size, regional shares, competitors with a rotavirus prophylaxis market share, detailed rotavirus prophylaxis market segments, market trends and opportunities and any further data you may need to thrive in the rotavirus prophylaxis industry. This rotavirus prophylaxis market research report delivers a complete perspective of everything you need, with an in-depth analysis of the current and future scenario of the industry.
The market value is defined as the revenues that enterprises gain from the sale of goods and/or services within the specified market and geography through sales, grants, or donations in terms of the currency (in USD unless otherwise specified).
The revenues for a specified geography are consumption values that are revenues generated by organizations in the specified geography within the market, irrespective of where they are produced. It does not include revenues from resales along the supply chain, either further along the supply chain or as part of other products.
This product will be delivered within 3-5 business days.
Table of Contents
Executive Summary
Rotavirus Prophylaxis Global Market Report 2025 provides strategists, marketers and senior management with the critical information they need to assess the market.This report focuses on rotavirus prophylaxis market which is experiencing strong growth. The report gives a guide to the trends which will be shaping the market over the next ten years and beyond.
Reasons to Purchase:
- Gain a truly global perspective with the most comprehensive report available on this market covering 15 geographies.
- Assess the impact of key macro factors such as conflict, pandemic and recovery, inflation and interest rate environment and the 2nd Trump presidency.
- Create regional and country strategies on the basis of local data and analysis.
- Identify growth segments for investment.
- Outperform competitors using forecast data and the drivers and trends shaping the market.
- Understand customers based on the latest market shares.
- Benchmark performance against key competitors.
- Suitable for supporting your internal and external presentations with reliable high quality data and analysis
- Report will be updated with the latest data and delivered to you along with an Excel data sheet for easy data extraction and analysis.
- All data from the report will also be delivered in an excel dashboard format.
Description
Where is the largest and fastest growing market for rotavirus prophylaxis? How does the market relate to the overall economy, demography and other similar markets? What forces will shape the market going forward? The rotavirus prophylaxis market global report answers all these questions and many more.The report covers market characteristics, size and growth, segmentation, regional and country breakdowns, competitive landscape, market shares, trends and strategies for this market. It traces the market’s historic and forecast market growth by geography.
- The market characteristics section of the report defines and explains the market.
- The market size section gives the market size ($b) covering both the historic growth of the market, and forecasting its development.
- The forecasts are made after considering the major factors currently impacting the market. These include:
- The forecasts are made after considering the major factors currently impacting the market. These include the Russia-Ukraine war, rising inflation, higher interest rates, and the legacy of the COVID-19 pandemic.
- Market segmentations break down the market into sub markets.
- The regional and country breakdowns section gives an analysis of the market in each geography and the size of the market by geography and compares their historic and forecast growth. It covers the growth trajectory of COVID-19 for all regions, key developed countries and major emerging markets.
- The competitive landscape chapter gives a description of the competitive nature of the market, market shares, and a description of the leading companies. Key financial deals which have shaped the market in recent years are identified.
- The trends and strategies section analyses the shape of the market as it emerges from the crisis and suggests how companies can grow as the market recovers.
Scope
Markets Covered:
1) By Treatment: Rotarix; Rotavac; Rotavin-M1; Oral Rehydration Fluid; Other Treatments2) By Route of Administration: Oral; Parenteral; Other Route of Administrations
3) By Distribution Channel: Hospital Pharmacy; Retail Pharmacy; Online Pharmacy; Other Distribution Channels
4) By End-Users: Hospitals; Specialty Clinics; Homecare; Other End-Users
Subsegments:
1) By Rotarix: Single Dose; Multi-Dose2) By Rotavac: Liquid Formulation; Freeze-Dried Formulation
3) By Rotavin-M1: Liquid Formulation; Freeze-Dried Formulation
4) By Oral Rehydration Fluid: Electrolyte Solutions; Glucose-Based Solutions
5) By Other Treatments: Supportive Care; Antidiarrheal Medications
Key Companies Mentioned: Pfizer Inc.; Johnson & Johnson; Merck & Co.; Novartis AG; Sanofi SA
Countries: Australia; Brazil; China; France; Germany; India; Indonesia; Japan; Russia; South Korea; UK; USA; Canada; Italy; Spain
Regions: Asia-Pacific; Western Europe; Eastern Europe; North America; South America; Middle East; Africa
Time Series: Five years historic and ten years forecast.
Data: Ratios of market size and growth to related markets, GDP proportions, expenditure per capita.
Data Segmentation: Country and regional historic and forecast data, market share of competitors, market segments.
Sourcing and Referencing: Data and analysis throughout the report is sourced using end notes.
Delivery Format: PDF, Word and Excel Data Dashboard.
Companies Mentioned
Some of the major companies featured in this Rotavirus Prophylaxis market report include:- Pfizer Inc.
- Johnson & Johnson
- Merck & Co.
- Novartis AG
- Sanofi SA
- Astra Zeneca Pharmaceuticals LP
- GlaxoSmithKline plc
- Takeda Pharmaceutical Company Limited
- Sinovac Biotech Ltd.
- Astellas Pharma Inc.
- CSL Limited
- Bharat Biotech International Limited
- Daiichi Sankyo Co. Ltd.
- Lupin Limited
- Mitsubishi Tanabe Pharma Corporation
- Serum Institute of India Pvt. Ltd.
- Zydus Lifescience Ltd.
- Emergent BioSolutions Inc.
- Hualan Biological Engineering Inc.
- Biocad Biopharmaceutical Co.
- Bharat Serums and Vaccines Limited
- Biological E Limited
- PT Bio Farma
- Panacea Biotec Ltd.
- Shanghai Institute of Biological Products (SIBP)
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 200 |
| Published | March 2025 |
| Forecast Period | 2025 - 2029 |
| Estimated Market Value ( USD | $ 10.03 Billion |
| Forecasted Market Value ( USD | $ 14.63 Billion |
| Compound Annual Growth Rate | 9.9% |
| Regions Covered | Global |
| No. of Companies Mentioned | 26 |