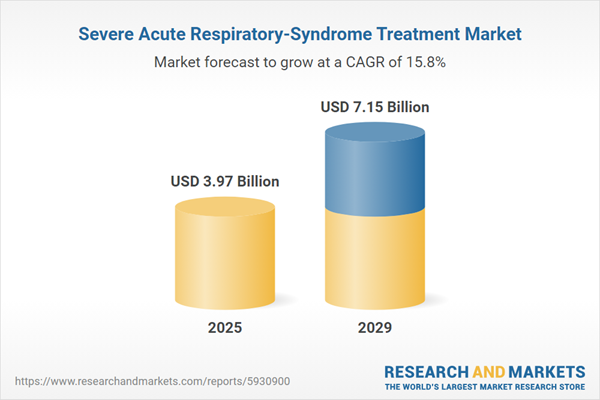
The severe acute respiratory-syndrome treatment market size has grown rapidly in recent years. It will grow from $3.45 billion in 2024 to $3.97 billion in 2025 at a compound annual growth rate (CAGR) of 15.2%. The growth in the historic period can be attributed to global outbreak response, antiviral drug development, vaccine development, public health preparedness.
The severe acute respiratory-syndrome treatment market size is expected to see rapid growth in the next few years. It will grow to $7.15 billion in 2029 at a compound annual growth rate (CAGR) of 15.8%. The growth in the forecast period can be attributed to broad-spectrum antivirals, emerging therapeutics, vaccine advancements, pandemic preparedness. Major trends in the forecast period include rna-targeted therapies, ai in drug discovery, global collaborations, public health education.
The increasing prevalence of respiratory diseases is expected to drive the growth of the severe acute respiratory syndrome (SARS) treatment market in the coming years. Respiratory diseases encompass a range of conditions that affect the respiratory system, which is responsible for breathing and facilitating gas exchange between the body and the environment. Treatments for severe acute respiratory syndrome aim to alleviate symptoms, prevent respiratory failure, reduce inflammation, and maintain oxygen levels. For example, in November 2023, the National Asthma Council, an Australia-based non-profit organization focused on asthma care, reported 467 asthma-related deaths in 2022, including 299 females and 168 males. This marked an increase from the 355 deaths recorded in 2021. Therefore, the rising incidence of respiratory diseases is fueling the growth of the severe acute respiratory syndrome treatment market.
The progressive surge in investments in research and development is poised to be a catalyst for the growth of the severe acute respiratory syndrome treatment market in the near future. These investments in research and development, often abbreviated as R&D, pertain to the allocation of financial resources and human expertise by organizations, governments, or entities with the aim of conducting systematic and innovative investigations and initiatives, with the goal of unearthing fresh knowledge. Augmented R&D investments play a pivotal role in advancing the array of available treatment options for severe acute respiratory syndromes, including conditions like SARS and COVID-19. Such investments contribute to the formulation of effective drugs, vaccines, diagnostic tools, and therapeutic strategies, ultimately enhancing patient outcomes and reinforcing global health preparedness. Notably, in August 2023, the United States Department of Health and Human Services, the executive branch department of the U.S. federal government, disclosed that the U.S. Department of Health and Human Services (HHS), in collaboration with the Administration for Strategic Preparedness and Response (ASPR), had allocated over $1.4 billion for Project NextGen. This initiative aims to support the development of the next generation of COVID-19 vaccines and treatments. Consequently, the increasing investments in research and development are the pivotal driving force behind the growth of the severe acute respiratory syndrome treatment market.
Leading companies actively participating in the severe acute respiratory syndrome treatment market are at the forefront of introducing innovative techniques, with a notable example being multiplexed real-time PCR. Multiplexed real-time PCR is a molecular biology technology that facilitates the simultaneous amplification and detection of multiple DNA or RNA targets in a single procedure. A case in point is the product launched by TransGen Biotech Co. Ltd., a Beijing-based manufacturer and researcher of molecular and cellular biology products, in July 2022. They introduced the Trans-SARS-CoV-2, Influenza A and B, and Respiratory Syncytial Virus Assay. This groundbreaking assay is designed as a multiplexed real-time PCR (polymerase chain reaction) assay, capable of detecting and distinguishing severe acute respiratory syndrome COVID-19 2 (SARS-CoV-2), Influenza A, Influenza B, and respiratory syncytial virus (RSV) in a single specimen. This innovative tool plays a crucial role in the diagnosis and treatment of severe acute respiratory syndromes, including COVID-19, influenza, and RSV, thus aiding in guiding treatment decisions and preventing the spread of these diseases.
In March 2022, AbbVie Inc., a U.S.-based pharmaceutical company, established a collaboration with Scripps Research, an independent, non-profit biomedical research and drug discovery institute also based in the United States. This strategic partnership is geared toward the development of cutting-edge direct-acting antiviral therapies designed for the treatment of COVID-19. The collaboration aims to pioneer innovative solutions for combatting the virus.
Major companies operating in the severe acute respiratory-syndrome treatment market are Pfizer Inc., F. Hoffmann-La Roche Ltd., AbbVie Inc., Merck And Co Inc., Novartis AG, Bristol Myers Squib company, GlaxoSmithKline PLC, AstraZeneca PLC, Sanofi SA, Eli Lilly And Company, Gilead Sciences Inc., Amgen Inc., Johnson And Johnson Private Limited, Moderna Inc., Regeneron Pharmaceuticals Inc., Hetero labs Ltd., Alexion Pharmaceuticals Inc., Cadila healthcare Ltd., Genentech Inc., Cipla Ltd., Biogen Inc., Vir Biotechnology Inc., Swedish Orphan Biovitrum AB, Dynavax Technologies Corporation, ViiV Healthcare Limited, Panacea Biotec Limited, CureVac N.V., CN Bio Innovations Ltd., Inovio Pharmaceuticals Inc., Chimerix Inc.
North America was the largest region in the severe acute respiratory syndrome treatment market in 2024. Western Europe is expected to be the fastest-growing region in the forecast period. The regions covered in severe acute respiratory syndrome treatment report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East and Africa. The countries covered in the severe acute respiratory syndrome treatment market report are Australia, Brazil, China, France, Germany, India, Indonesia, Japan, Russia, South Korea, UK, USA, Canada, Italy, Spain.
The severe acute respiratory syndrome treatment market consists of revenues earned by entities by providing services such as respiratory therapy, oxygen therapy, ventilation, intensive care, isolation and infection control, fluid and electrolyte management and monitoring services. The market value includes the value of related goods sold by the service provider or included within the service offering. The severe acute respiratory syndrome treatment market also includes sales of azithromycin, immunomodulators, vaccines, ribavirin, ritonavir, remdesivir, methylprednisolone, prednisolone, dexamethasone and interferon. Values in this market are ‘factory gate’ values, that is, the value of goods sold by the manufacturers or creators of the goods, whether to other entities (including downstream manufacturers, wholesalers, distributors and retailers) or directly to end customers. The value of goods in this market includes related services sold by the creators of the goods.
Severe acute respiratory syndrome (SARS) is a severe type of pneumonia caused by an airborne virus that can be transmitted through tiny droplets of saliva. This previously unidentified virus belongs to the Coronaviridae family and is known as SARS-associated COVID-19 (SARS-CoV). The treatment for severe acute respiratory syndrome (SARS) involves medical interventions and supportive measures aimed at managing the symptoms and complications of this viral respiratory illness.
The primary classes of drugs used in the treatment of severe acute respiratory syndrome include antibiotics, antivirals, corticosteroids, monoclonal antibodies, and others. Antibiotics are a class of medications used to treat bacterial infections. They function by either killing bacteria or inhibiting their growth, depending on the specific type of antibiotic. In the context of severe acute respiratory syndrome caused by SARS-CoV and SARS-CoV-2, these antibiotics are administered orally and intravenously. They are distributed through various channels, including hospital pharmacies and retail pharmacies, among others.
The severe acute respiratory syndrome treatment market research report is one of a series of new reports that provides severe acute respiratory-syndrome treatment market statistics, including severe acute respiratory-syndrome treatment industry global market size, regional shares, competitors with a severe acute respiratory syndrome treatment market share, detailed severe acute respiratory-syndrome treatment market segments, market trends and opportunities and any further data you may need to thrive in the severe acute respiratory-syndrome treatment industry. This severe acute respiratory-syndrome treatment market research report delivers a complete perspective of everything you need, with an in-depth analysis of the current and future scenarios of the industry.
The market value is defined as the revenues that enterprises gain from the sale of goods and/or services within the specified market and geography through sales, grants, or donations in terms of the currency (in USD unless otherwise specified).
The revenues for a specified geography are consumption values that are revenues generated by organizations in the specified geography within the market, irrespective of where they are produced. It does not include revenues from resales along the supply chain, either further along the supply chain or as part of other products.
This product will be delivered within 3-5 business days.
Table of Contents
Executive Summary
Severe Acute Respiratory-Syndrome Treatment Global Market Report 2025 provides strategists, marketers and senior management with the critical information they need to assess the market.This report focuses on severe acute respiratory-syndrome treatment market which is experiencing strong growth. The report gives a guide to the trends which will be shaping the market over the next ten years and beyond.
Reasons to Purchase:
- Gain a truly global perspective with the most comprehensive report available on this market covering 15 geographies.
- Assess the impact of key macro factors such as conflict, pandemic and recovery, inflation and interest rate environment and the 2nd Trump presidency.
- Create regional and country strategies on the basis of local data and analysis.
- Identify growth segments for investment.
- Outperform competitors using forecast data and the drivers and trends shaping the market.
- Understand customers based on the latest market shares.
- Benchmark performance against key competitors.
- Suitable for supporting your internal and external presentations with reliable high quality data and analysis
- Report will be updated with the latest data and delivered to you along with an Excel data sheet for easy data extraction and analysis.
- All data from the report will also be delivered in an excel dashboard format.
Description
Where is the largest and fastest growing market for severe acute respiratory-syndrome treatment? How does the market relate to the overall economy, demography and other similar markets? What forces will shape the market going forward? The severe acute respiratory-syndrome treatment market global report answers all these questions and many more.The report covers market characteristics, size and growth, segmentation, regional and country breakdowns, competitive landscape, market shares, trends and strategies for this market. It traces the market’s historic and forecast market growth by geography.
- The market characteristics section of the report defines and explains the market.
- The market size section gives the market size ($b) covering both the historic growth of the market, and forecasting its development.
- The forecasts are made after considering the major factors currently impacting the market. These include:
- The forecasts are made after considering the major factors currently impacting the market. These include the Russia-Ukraine war, rising inflation, higher interest rates, and the legacy of the COVID-19 pandemic.
- Market segmentations break down the market into sub markets.
- The regional and country breakdowns section gives an analysis of the market in each geography and the size of the market by geography and compares their historic and forecast growth. It covers the growth trajectory of COVID-19 for all regions, key developed countries and major emerging markets.
- The competitive landscape chapter gives a description of the competitive nature of the market, market shares, and a description of the leading companies. Key financial deals which have shaped the market in recent years are identified.
- The trends and strategies section analyses the shape of the market as it emerges from the crisis and suggests how companies can grow as the market recovers.
Scope
Markets Covered:
1) By Drug Class: Antibiotics; Antiviral; Corticosteroids; Monoclonal Antibodies; Other Drug Classes2) By Route Of Administration: Oral; Intravenous
3) By Indication: Severe Acute Respiratory Syndrome COVID-19 (SARS-CoV); Severe Acute Respiratory Syndrome COVID-19 2 (SARS‑CoV‑2)
4) By Distribution Channel: Hospital Pharmacies; Retail Pharmacies; Other Distribution Channels
Subsegments:
1) By Antibiotics: Broad-Spectrum Antibiotics; Narrow-Spectrum Antibiotics2) By Antiviral: Nucleoside Analogs; Protease Inhibitors
3) By Corticosteroids: Systemic Corticosteroids; Inhaled Corticosteroids
4) By Monoclonal Antibodies: Neutralizing Antibodies; Non-Neutralizing Antibodies
5) By Other Drug Classes: Immunomodulators; Supportive Medications
Key Companies Mentioned: Pfizer Inc.; F. Hoffmann-La Roche Ltd.; AbbVie Inc.; Merck And Co Inc.; Novartis AG
Countries: Australia; Brazil; China; France; Germany; India; Indonesia; Japan; Russia; South Korea; UK; USA; Canada; Italy; Spain
Regions: Asia-Pacific; Western Europe; Eastern Europe; North America; South America; Middle East; Africa
Time Series: Five years historic and ten years forecast.
Data: Ratios of market size and growth to related markets, GDP proportions, expenditure per capita.
Data Segmentation: Country and regional historic and forecast data, market share of competitors, market segments.
Sourcing and Referencing: Data and analysis throughout the report is sourced using end notes.
Delivery Format: PDF, Word and Excel Data Dashboard.
Companies Mentioned
Some of the major companies featured in this Severe Acute Respiratory-Syndrome Treatment market report include:- Pfizer Inc.
- F. Hoffmann-La Roche Ltd.
- AbbVie Inc.
- Merck And Co Inc.
- Novartis AG
- Bristol Myers Squib company
- GlaxoSmithKline plc
- AstraZeneca plc
- Sanofi SA
- Eli Lilly And Company
- Gilead Sciences Inc.
- Amgen Inc.
- Johnson And Johnson Private Limited
- Moderna Inc.
- Regeneron Pharmaceuticals Inc.
- Hetero labs Ltd.
- Alexion Pharmaceuticals Inc.
- Cadila healthcare Ltd.
- Genentech Inc.
- Cipla Ltd.
- Biogen Inc.
- Vir Biotechnology Inc.
- Swedish Orphan Biovitrum AB
- Dynavax Technologies Corporation
- ViiV Healthcare Limited
- Panacea Biotec Limited
- CureVac N.V.
- CN Bio Innovations Ltd.
- Inovio Pharmaceuticals Inc.
- Chimerix Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 200 |
| Published | March 2025 |
| Forecast Period | 2025 - 2029 |
| Estimated Market Value ( USD | $ 3.97 Billion |
| Forecasted Market Value ( USD | $ 7.15 Billion |
| Compound Annual Growth Rate | 15.8% |
| Regions Covered | Global |
| No. of Companies Mentioned | 30 |