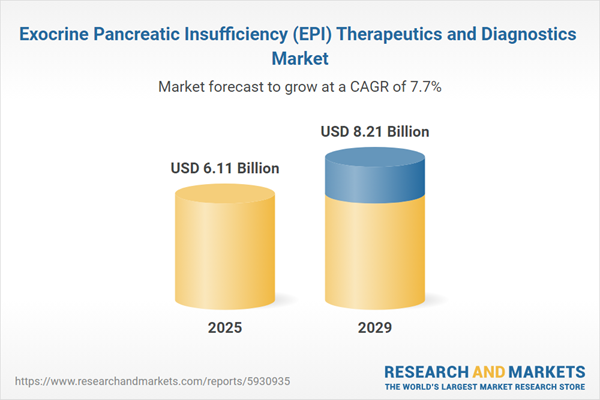
The exocrine pancreatic insufficiency (epi) therapeutics and diagnostics market size is expected to see strong growth in the next few years. It will grow to $8.21 billion in 2029 at a compound annual growth rate (CAGR) of 7.7%. The growth in the forecast period can be attributed to telemedicine and remote monitoring, data analytics and ai, patient education and support, regulatory changes and reimbursement policies. Major trends in the forecast period include personalized treatment approaches, development of novel therapies, minimally invasive diagnostics, collaborative care models.
The forecast of 7.7% growth over the next five years reflects a slight reduction of 0.1% from the previous projection. This reduction is primarily due to the impact of tariffs between the US and other countries. The imposition of tariffs could disrupt U.S. gastroenterology practices by increasing prices of pancreatic enzyme replacements and fecal elastase test kits sourced from France and India, exacerbating chronic pancreatitis and cystic fibrosis management costs. The effect will also be felt more widely due to reciprocal tariffs and the negative effect on the global economy and trade due to increased trade tensions and restrictions.
The growing prevalence of diabetes is anticipated to drive the expansion of the exocrine pancreatic insufficiency (EPI) therapeutics and diagnostics market in the future. Diabetes is a chronic condition characterized by elevated blood sugar levels (hyperglycemia), resulting from the body's inability to produce sufficient insulin or use the insulin effectively. EPI treatment, including pancreatic enzyme replacement therapy (PERT), can enhance nutrient absorption and support better glycemic control in individuals with both diabetes and EPI. For example, in June 2024, the National Health Service (NHS England) reported that the number of people diagnosed with pre-diabetes in 2023 increased to 3,615,330, an 18% rise from 3,065,825 in 2022. Among those under 40, pre-diabetes cases rose nearly 25%, from 173,166 in 2022 to 216,440 in 2023. This growing diabetic population is fueling the demand for EPI therapeutics and diagnostics.
The surge in cases of gastrointestinal disorders is projected to drive the expansion of the exocrine pancreatic insufficiency (EPI) therapeutics and diagnostics market in the foreseeable future. Gastrointestinal disorders encompass a range of medical conditions affecting the digestive tract, including the organs responsible for digestion. Managing gastrointestinal diseases relies significantly on exocrine pancreatic insufficiency (EPI) therapeutics and diagnostics, which rectify enzyme deficiencies and promote effective nutrient digestion. For instance, as of June 2023, Crohn's and Colitis Canada, a non-profit organization based in Canada, estimated that over 322,600 Canadians were living with inflammatory bowel diseases (IBD) in 2023, constituting about 0.82% of the population. With the prevalence of IBD projected to persist, it is anticipated that approximately 470,000 Canadians will have IBD by 2035, roughly 1.1% of the population, or about 1 in every 91 individuals in the country. Consequently, the escalating incidence of gastrointestinal disorders is anticipated to be a driving force behind the growth of the exocrine pancreatic insufficiency (EPI) therapeutics and diagnostics market.
Key players in the exocrine pancreatic insufficiency (EPI) therapeutics and diagnostics market are increasingly forming strategic partnerships to integrate advanced technologies and broaden their market presence. A strategic partnership involves collaboration between two or more organizations, combining their resources, expertise, and efforts to reach shared objectives. For example, in February 2023, Codexis, Inc., a protein engineering firm based in the US, partnered with Nestlé Health Science, a Swiss nutrition company, to announce the interim results of their Phase 1 clinical trial of CDX-7108, an investigational therapy for EPI. These promising results from the trial open the door for further research that could lead to improved treatment options for individuals affected by EPI.
In October 2022, DiaSorin SpA, an Italian biotechnology firm, acquired Luminex Corporation for $1.8 billion. This acquisition allows DiaSorin to bolster its presence in the U.S. market, expand its product portfolio, and establish long-term collaborations with biopharmaceutical companies. Luminex Corporation, based in the U.S., specializes in biological testing technologies, including those related to exocrine pancreatic insufficiency (EPI).
Major companies operating in the exocrine pancreatic insufficiency (epi) therapeutics and diagnostics market are Pfizer Inc., Merck & Co. Inc., Janssen Pharmaceuticals Inc., AbbVie Inc., Novartis AG, Sanofi S.A., AstraZeneca PLC, Abott Laboratories, Medtronic PLC, Solvay S.A., Laboratory Corporation of America Holdings (Labcorp), Organon group of companies Metagenics LLC, Allergan PLC, Chiesi Farmaceutici S.p.A., Codexis Inc., Nordmark Arzneimittel GmbH & Co. KG, ChiRhoClin Inc., Digestive Care Inc., Alcresta Therapeutics Inc., Vivus Inc., Cilian AG, Bioserv Diagnostics Gmbh, Anthera Pharmaceuticals Inc., Aptalis Pharma Inc., First Wave BioPharma Inc., EagleBiosciences Inc., ScheBo Biotech AG.
North America was the largest region in the exocrine pancreatic insufficiency (EPI) therapeutics and diagnostics market in 2024. The regions covered in exocrine pancreatic insufficiency (EPI) therapeutics and diagnostics report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East and Africa. The countries covered in the exocrine pancreatic insufficiency (EPI) therapeutics and diagnostics market report are Australia, Brazil, China, France, Germany, India, Indonesia, Japan, Russia, South Korea, UK, USA, Canada, Italy, Spain.
Note that the outlook for this market is being affected by rapid changes in trade relations and tariffs globally. The report will be updated prior to delivery to reflect the latest status, including revised forecasts and quantified impact analysis. The report’s Recommendations and Conclusions sections will be updated to give strategies for entities dealing with the fast-moving international environment.
The sudden escalation of U.S. tariffs and the resulting trade tensions in spring 2025 are having a significant impact on the pharmaceutical sector. Companies are grappling with higher costs on imported active pharmaceutical ingredients (APIs), glass vials, and laboratory equipment - many of which have limited alternative sources. Generic drug manufacturers, already operating with minimal profit margins, are particularly affected, with some scaling back production of low-margin medications. Biotech firms are also experiencing delays in clinical trials due to shortages of specialized reagents linked to tariffs. In response, the industry is shifting API production to regions like India and Europe, building up inventory reserves, and advocating for tariff exemptions on essential medicines.
The exocrine pancreatic insufficiency (EPI) therapeutics and diagnostics market research report is one of a series of new reports that provides exocrine pancreatic insufficiency (EPI) therapeutics and diagnostics market statistics, including exocrine pancreatic insufficiency (EPI) therapeutics and diagnostics industry global market size, regional shares, competitors with a exocrine pancreatic insufficiency (EPI) therapeutics and diagnostics market share, detailed exocrine pancreatic insufficiency (EPI) therapeutics and diagnostics market segments, market trends and opportunities and any further data you may need to thrive in the exocrine pancreatic insufficiency (EPI) therapeutics and diagnostics industry. This exocrine pancreatic insufficiency (EPI) therapeutics and diagnostics market research report delivers a complete perspective of everything you need, with an in-depth analysis of the current and future scenario of the industry.
Exocrine pancreatic insufficiency (EPI) therapeutics and diagnostics pertain to the treatment of a condition where the pancreas fails to produce adequate digestive enzymes necessary for the effective breakdown and absorption of nutrients from food within the small intestine. The role of EPI diagnostics and therapeutics is pivotal in the identification and management of exocrine pancreatic insufficiency, facilitating individuals with EPI in attaining improved nutrient absorption and overall well-being.
The primary forms of treatments encompassed within exocrine pancreatic insufficiency (EPI) therapeutics and diagnostics comprise nutritional management, pancreatic enzyme replacement therapy (PERT), and lifestyle adjustments. Nutritional management is a specialized healthcare approach utilizing nutrition and diet to address various medical and health conditions. Diverse diagnostic techniques are employed, including blood tests, magnetic resonance imaging (MRI), endoscopic ultrasonography (EUS), and computerized tomography (CT) scanning. Various types of medications, both generic and branded, are utilized, and these are distributed through a range of channels such as direct tender, retail pharmacy, third-party distributor, and others. These are availed by a diverse array of end users including hospitals, specialty clinics, homecare, diagnostic centers, research and academic institutes, among others.
The exocrine pancreatic insufficiency (EPI) therapeutics and diagnostics market consists of revenues earned by entities by providing dietary interventions and bile acid replacement therapy (BAR). The market value includes the value of related goods sold by the service provider or included within the service offering. The exocrine pancreatic insufficiency (EPI) therapeutics and diagnostics market also includes sales of Creon, Zenpep and Ultresa. Values in this market are ‘factory gate’ values, that is the value of goods sold by the manufacturers or creators of the goods, whether to other entities (including downstream manufacturers, wholesalers, distributors and retailers) or directly to end customers. The value of goods in this market includes related services sold by the creators of the goods.
The market value is defined as the revenues that enterprises gain from the sale of goods and/or services within the specified market and geography through sales, grants, or donations in terms of the currency (in USD, unless otherwise specified).
The revenues for a specified geography are consumption values that are revenues generated by organizations in the specified geography within the market, irrespective of where they are produced. It does not include revenues from resales along the supply chain, either further along the supply chain or as part of other products.
This product will be delivered within 1-3 business days.
Table of Contents
Executive Summary
Exocrine Pancreatic Insufficiency (EPI) Therapeutics and Diagnostics Global Market Report 2025 provides strategists, marketers and senior management with the critical information they need to assess the market.This report focuses on exocrine pancreatic insufficiency (epi) therapeutics and diagnostics market which is experiencing strong growth. The report gives a guide to the trends which will be shaping the market over the next ten years and beyond.
Reasons to Purchase:
- Gain a truly global perspective with the most comprehensive report available on this market covering 15 geographies.
- Assess the impact of key macro factors such as geopolitical conflicts, trade policies and tariffs, post-pandemic supply chain realignment, inflation and interest rate fluctuations, and evolving regulatory landscapes.
- Create regional and country strategies on the basis of local data and analysis.
- Identify growth segments for investment.
- Outperform competitors using forecast data and the drivers and trends shaping the market.
- Understand customers based on the latest market shares.
- Benchmark performance against key competitors.
- Suitable for supporting your internal and external presentations with reliable high quality data and analysis
- Report will be updated with the latest data and delivered to you along with an Excel data sheet for easy data extraction and analysis.
- All data from the report will also be delivered in an excel dashboard format.
Description
Where is the largest and fastest growing market for exocrine pancreatic insufficiency (epi) therapeutics and diagnostics? How does the market relate to the overall economy, demography and other similar markets? What forces will shape the market going forward, including technological disruption, regulatory shifts, and changing consumer preferences? The exocrine pancreatic insufficiency (epi) therapeutics and diagnostics market global report answers all these questions and many more.The report covers market characteristics, size and growth, segmentation, regional and country breakdowns, competitive landscape, market shares, trends and strategies for this market. It traces the market’s historic and forecast market growth by geography.
- The market characteristics section of the report defines and explains the market.
- The market size section gives the market size ($b) covering both the historic growth of the market, and forecasting its development.
- The forecasts are made after considering the major factors currently impacting the market. These include: the technological advancements such as AI and automation, Russia-Ukraine war, trade tariffs (government-imposed import/export duties), elevated inflation and interest rates.
- Market segmentations break down the market into sub markets.
- The regional and country breakdowns section gives an analysis of the market in each geography and the size of the market by geography and compares their historic and forecast growth.
- The competitive landscape chapter gives a description of the competitive nature of the market, market shares, and a description of the leading companies. Key financial deals which have shaped the market in recent years are identified.
- The trends and strategies section analyses the shape of the market as it emerges from the crisis and suggests how companies can grow as the market recovers.
Scope
Markets Covered:
1) By Therapies: Nutritional Management; Pancreatic Enzyme Replacement Therapy (PERT); Lifestyle Modifications Approach2) By Diagnostics: Blood Tests; Magnetic Resonance Imaging (MRI); Endoscopic Ultra-Sonography (EUS); Computerized Tomography (CT) Scanning
3) By Drug Type: Generic; Branded
4) By Distribution Channel: Direct Tender; Retail Pharmacy ; Third-Party Distributor; Other Distribution Channels
5) By End User: Hospitals; Specialty Clinics; Homecare; Diagnostic Center; Research and Academic Institutes; Other End Users
Subsegments:
1) By Nutritional Management: Dietary Modifications; Nutritional Supplements2) By Pancreatic Enzyme Replacement Therapy (PERT): Capsule Formulations; Tablet Formulations; Powder Formulations
3) By Lifestyle Modifications Approach: Weight Management; Physical Activity Recommendations; Smoking Cessation Programs
Companies Mentioned: Pfizer Inc.; Merck & Co. Inc.; Janssen Pharmaceuticals Inc.; AbbVie Inc.; Novartis AG; Sanofi S.A.; AstraZeneca plc; Abott Laboratories; Medtronic plc; Solvay S.A.; Laboratory Corporation of America Holdings (Labcorp); Organon group of companies Metagenics LLC; Allergan plc; Chiesi Farmaceutici S.p.A.; Codexis Inc.; Nordmark Arzneimittel GmbH & Co. KG; ChiRhoClin Inc.; Digestive Care Inc.; Alcresta Therapeutics Inc.; Vivus Inc.; Cilian AG; Bioserv Diagnostics Gmbh; Anthera Pharmaceuticals Inc.; Aptalis Pharma Inc.; First Wave BioPharma Inc.; EagleBiosciences Inc.; ScheBo Biotech AG
Countries: Australia; Brazil; China; France; Germany; India; Indonesia; Japan; Russia; South Korea; UK; USA; Canada; Italy; Spain
Regions: Asia-Pacific; Western Europe; Eastern Europe; North America; South America; Middle East; Africa
Time Series: Five years historic and ten years forecast.
Data: Ratios of market size and growth to related markets, GDP proportions, expenditure per capita.
Data Segmentation: Country and regional historic and forecast data, market share of competitors, market segments.
Sourcing and Referencing: Data and analysis throughout the report is sourced using end notes.
Delivery Format: PDF, Word and Excel Data Dashboard.
Companies Mentioned
The companies featured in this Exocrine Pancreatic Insufficiency (EPI) Therapeutics and Diagnostics market report include:- Pfizer Inc.
- Merck & Co. Inc.
- Janssen Pharmaceuticals Inc.
- AbbVie Inc.
- Novartis AG
- Sanofi S.A.
- AstraZeneca plc
- Abott Laboratories
- Medtronic plc
- Solvay S.A.
- Laboratory Corporation of America Holdings (Labcorp)
- Organon group of companies Metagenics LLC
- Allergan plc
- Chiesi Farmaceutici S.p.A.
- Codexis Inc.
- Nordmark Arzneimittel GmbH & Co. KG
- ChiRhoClin Inc.
- Digestive Care Inc.
- Alcresta Therapeutics Inc.
- Vivus Inc.
- Cilian AG
- Bioserv Diagnostics Gmbh
- Anthera Pharmaceuticals Inc.
- Aptalis Pharma Inc.
- First Wave BioPharma Inc.
- EagleBiosciences Inc.
- ScheBo Biotech AG
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 250 |
| Published | September 2025 |
| Forecast Period | 2025 - 2029 |
| Estimated Market Value ( USD | $ 6.11 Billion |
| Forecasted Market Value ( USD | $ 8.21 Billion |
| Compound Annual Growth Rate | 7.7% |
| Regions Covered | Global |
| No. of Companies Mentioned | 27 |