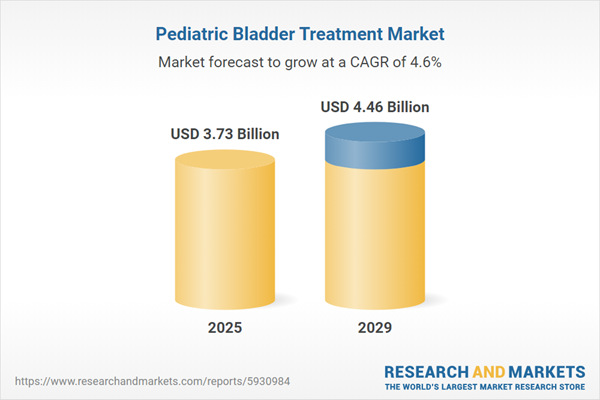
The pediatric bladder treatment market size has grown strongly in recent years. It will grow from $3.54 billion in 2024 to $3.73 billion in 2025 at a compound annual growth rate (CAGR) of 5.1%. The growth in the historic period can be attributed to prevalence of pediatric bladder conditions, growing awareness of pediatric bladder conditions, increasing access to healthcare for children, rising disposable incomes.
The pediatric bladder treatment market size is expected to see steady growth in the next few years. It will grow to $4.46 billion in 2029 at a compound annual growth rate (CAGR) of 4.6%. The growth in the forecast period can be attributed to aging population, rising prevalence of chronic diseases in children, increasing access to healthcare, demand for personalized medicine in the treatment. Major trends in the forecast period include adoption of new and innovative pediatric bladder treatments, digital health technologies, early intervention and prevention, non-invasive treatments.
The rising prevalence of pediatric bladder disorders is poised to fuel the expansion of the pediatric bladder treatment market in the foreseeable future. Pediatric bladder disorders encompass a range of medical conditions that disrupt the normal functionality of the urinary bladder in children. These conditions, often associated with ailments like spina bifida and neurological disorders, result in urinary complications in children, necessitating effective treatment approaches. For example, a study involving 1,065 pediatric patients with neurogenic bladder (NGB, a type of pediatric bladder disorder), conducted in March 2023 and published by the Multidisciplinary Digital Publishing Institute (MDPI), revealed that 38.9% of these patients had spina bifida. Lower urinary tract infections were a prevalent complication (18.1%), especially among patients with open spina bifida (54.1%). Over a 12-month follow-up, common complications included recurrent lower urinary tract infections (18.1%), urinary incontinence (8%), and hydronephrosis (5.6%), with 7.9% of patients experiencing four or more recurring lower urinary tract infections. Consequently, the surge in pediatric bladder disorders is a key driver of growth in the pediatric bladder treatment market.
The growing acceptance of minimally invasive procedures is poised to revolutionize the pediatric bladder treatment market's growth in the coming years. Minimally invasive procedures encompass medical and surgical methods characterized by the use of small incisions, delicate instruments, and advanced imaging technology. The rising application of minimally invasive interventions in pediatric bladder treatment offers several advantages, including swifter recovery, reduced discomfort, and enhanced safety. These benefits position minimally invasive techniques as more effective and appropriate options for addressing pediatric bladder issues. For example, as reported by Intuitive Surgical Inc., a US-based manufacturer of robotic systems, the year 2022 witnessed around 1,875,000 surgical procedures carried out using Vinci Surgical Systems, signifying an impressive 18% surge in comparison to the approximately 1,594,000 surgical procedures conducted with Vinci Surgical Systems in 2021. As such, the increasing adoption of minimally invasive procedures drives the growth of the pediatric bladder treatment market.
In November 2023, NYU Langone Health, a medical center based in the U.S., acquired Pediatric Urology Associates for an undisclosed amount. Through this acquisition, Pediatric Urology Associates will merge with NYU Langone, tripling the size of its pediatric urology care team and adding 10 more practice locations. This integration will also facilitate the adoption of NYU Langone’s Epic electronic health record system, enhancing patient care coordination. Pediatric Urology Associates is a U.S.-based company specializing in treatments for pediatric bladder conditions.
Major companies operating in the pediatric bladder treatment market are Pfizer Inc., F. Hoffmann-La Roche Ltd., AbbVie Inc., Novartis AG, Sanofi S.A., AstraZeneca PLC, Takeda Pharmaceutical Company Limited, Medtronic PLC, Teva Pharmaceutical Industries Ltd., Boston Scientific Corporation, Mylan N.V., Astellas Pharma Inc., Bausch Health Companies Inc., Sun Pharmaceutical Industries Ltd., Cook Medical Inc., Ipsen Pharma, Aurobindo Pharma Ltd., Endo International PLC, Coloplast AIS, Endo International PLC, Apotex Inc., Amneal Pharmaceuticals LLC., Orion Corporation, Taro Pharmaceuticals, Laborie Medical Technologies Corp., Salix Pharmaceuticals, TherapeuticsMD Inc., UroGen Pharma, Palette Life Sciences, Sumitovant Biopharma.
North America was the largest region in the pediatric bladder treatment market in 2024. Asia-Pacific is expected to be the fastest-growing region in the forecast period. The regions covered in pediatric bladder treatment report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East and Africa. The countries covered in the pediatric bladder treatment market report are Australia, Brazil, China, France, Germany, India, Indonesia, Japan, Russia, South Korea, UK, USA, Canada, Italy, Spain.
The pediatric bladder treatment market consists of revenues earned by entities by providing medical consultations, nutritional counseling, catheterization and therapies. The market value includes the value of related goods sold by the service provider or included within the service offering. The pediatric bladder treatment market also includes sales of ultrasound devices, cystoscopes, antibiotics, urinary catheters, incontinence products and surgical instruments. Values in this market are ‘factory gate’ values, that is the value of goods sold by the manufacturers or creators of the goods, whether to other entities (including downstream manufacturers, wholesalers, distributors and retailers) or directly to end customers. The value of goods in this market includes related services sold by the creators of the goods.
The market value is defined as the revenues that enterprises gain from the sale of goods and/or services within the specified market and geography through sales, grants, or donations in terms of the currency (in USD unless otherwise specified).
Pediatric bladder disorders encompass a range of medical conditions that affect the urinary bladder in children, including issues related to bladder function, control, and structure. The treatment of pediatric bladder disorders involves the management and addressing of bladder dysfunction, with the choice of treatment methods contingent on factors like the child's age, overall health, medical history, specific bladder symptoms, and the underlying cause of nerve damage or neurological conditions.
The primary categories of pediatric bladder treatment for these disorders consist of diurnal incontinence, enuresis, and other related conditions. Diurnal incontinence pertains to the involuntary release of urine during daylight hours, when an individual should typically have control over their bladder. Treatment for diurnal incontinence in children often includes behavioral and lifestyle adjustments, such as educating the child to recognize and respond to the sensation of a full bladder, encouraging regular bathroom breaks, and addressing any issues related to constipation. These conditions are also managed through urotherapy, medication, surgical reconstruction, and other treatment methods, administered orally, parenterally, or through alternative routes. The treatment products are distributed through various channels, including hospital pharmacies, online pharmacies, and retail pharmacies, and cater to end users such as hospitals, homecare services, specialty clinics, and more.
The pediatric bladder treatment market research report is one of a series of new reports that provides pediatric bladder treatment market statistics, including pediatric bladder treatment industry global market size, regional shares, competitors with a pediatric bladder treatment market share, detailed pediatric bladder treatment market segments, market trends and opportunities and any further data you may need to thrive in the pediatric bladder treatment industry. This pediatric bladder treatment market research report delivers a complete perspective of everything you need, with an in-depth analysis of the current and future scenario of the industry.
The revenues for a specified geography are consumption values that are revenues generated by organizations in the specified geography within the market, irrespective of where they are produced. It does not include revenues from resales along the supply chain, either further along the supply chain or as part of other products.
This product will be delivered within 3-5 business days.
Table of Contents
Executive Summary
Pediatric Bladder Treatment Global Market Report 2025 provides strategists, marketers and senior management with the critical information they need to assess the market.This report focuses on pediatric bladder treatment market which is experiencing strong growth. The report gives a guide to the trends which will be shaping the market over the next ten years and beyond.
Reasons to Purchase:
- Gain a truly global perspective with the most comprehensive report available on this market covering 15 geographies.
- Assess the impact of key macro factors such as conflict, pandemic and recovery, inflation and interest rate environment and the 2nd Trump presidency.
- Create regional and country strategies on the basis of local data and analysis.
- Identify growth segments for investment.
- Outperform competitors using forecast data and the drivers and trends shaping the market.
- Understand customers based on the latest market shares.
- Benchmark performance against key competitors.
- Suitable for supporting your internal and external presentations with reliable high quality data and analysis
- Report will be updated with the latest data and delivered to you along with an Excel data sheet for easy data extraction and analysis.
- All data from the report will also be delivered in an excel dashboard format.
Description
Where is the largest and fastest growing market for pediatric bladder treatment? How does the market relate to the overall economy, demography and other similar markets? What forces will shape the market going forward? The pediatric bladder treatment market global report answers all these questions and many more.The report covers market characteristics, size and growth, segmentation, regional and country breakdowns, competitive landscape, market shares, trends and strategies for this market. It traces the market’s historic and forecast market growth by geography.
- The market characteristics section of the report defines and explains the market.
- The market size section gives the market size ($b) covering both the historic growth of the market, and forecasting its development.
- The forecasts are made after considering the major factors currently impacting the market. These include:
- The forecasts are made after considering the major factors currently impacting the market. These include the Russia-Ukraine war, rising inflation, higher interest rates, and the legacy of the COVID-19 pandemic.
- Market segmentations break down the market into sub markets.
- The regional and country breakdowns section gives an analysis of the market in each geography and the size of the market by geography and compares their historic and forecast growth. It covers the growth trajectory of COVID-19 for all regions, key developed countries and major emerging markets.
- The competitive landscape chapter gives a description of the competitive nature of the market, market shares, and a description of the leading companies. Key financial deals which have shaped the market in recent years are identified.
- The trends and strategies section analyses the shape of the market as it emerges from the crisis and suggests how companies can grow as the market recovers.
Scope
Markets Covered:
1) By Type: Diurnal Incontinence; Enuresis; Other Types2) By Treatment: Urotherapy; Medication; Surgical Reconstruction; Other Treatments
3) By Route of Administration: Oral; Parenteral; Other Route of Administrations
4) By Distribution Channel: Hospital Pharmacy; Online Pharmacy; Retail Pharmacy
5) By End User: Hospitals; Homecare; Specialty Clinics; Other End-Users
Subsegments:
1) By Diurnal Incontinence: Overactive Bladder; Urge Incontinence; Other Diurnal Incontinence Types2) By Enuresis: Primary Enuresis; Secondary Enuresis; Nocturnal Enuresis
3) By Other Types: Neurogenic Bladder; Structural Abnormalities; Other Pediatric Bladder Disorders
Key Companies Mentioned: Pfizer Inc.; F. Hoffmann-La Roche Ltd; AbbVie Inc.; Novartis AG; Sanofi S.A.
Countries: Australia; Brazil; China; France; Germany; India; Indonesia; Japan; Russia; South Korea; UK; USA; Canada; Italy; Spain
Regions: Asia-Pacific; Western Europe; Eastern Europe; North America; South America; Middle East; Africa
Time Series: Five years historic and ten years forecast.
Data: Ratios of market size and growth to related markets, GDP proportions, expenditure per capita.
Data Segmentation: Country and regional historic and forecast data, market share of competitors, market segments.
Sourcing and Referencing: Data and analysis throughout the report is sourced using end notes.
Delivery Format: PDF, Word and Excel Data Dashboard.
Companies Mentioned
Some of the major companies featured in this Pediatric Bladder Treatment market report include:- Pfizer Inc.
- F. Hoffmann-La Roche Ltd
- AbbVie Inc.
- Novartis AG
- Sanofi S.A.
- AstraZeneca Plc
- Takeda Pharmaceutical Company Limited
- Medtronic Plc
- Teva Pharmaceutical Industries Ltd.
- Boston Scientific Corporation
- Mylan N.V.
- Astellas Pharma Inc
- Bausch Health Companies Inc.
- Sun Pharmaceutical Industries Ltd.
- Cook Medical Inc.
- Ipsen Pharma
- Aurobindo Pharma Ltd
- Endo International plc
- Coloplast AIS
- Endo International plc
- Apotex Inc.
- Amneal Pharmaceuticals LLC.
- Orion Corporation
- Taro Pharmaceuticals
- Laborie Medical Technologies Corp.
- Salix Pharmaceuticals
- TherapeuticsMD Inc.
- UroGen Pharma
- Palette Life Sciences
- Sumitovant Biopharma.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 200 |
| Published | March 2025 |
| Forecast Period | 2025 - 2029 |
| Estimated Market Value ( USD | $ 3.73 Billion |
| Forecasted Market Value ( USD | $ 4.46 Billion |
| Compound Annual Growth Rate | 4.6% |
| Regions Covered | Global |
| No. of Companies Mentioned | 31 |