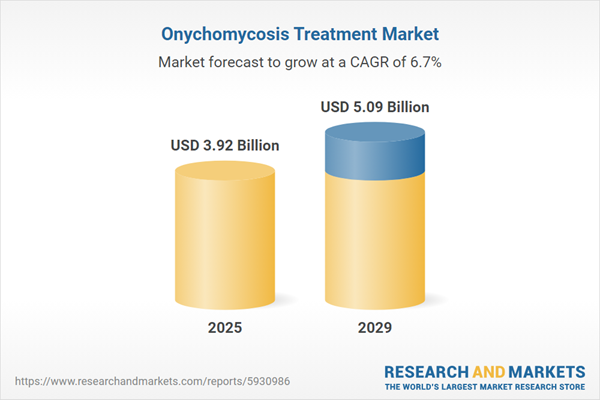
The onychomycosis treatment market size has grown strongly in recent years. It will grow from $3.65 billion in 2024 to $3.92 billion in 2025 at a compound annual growth rate (CAGR) of 7.4%. The growth in the historic period can be attributed to increasing fungal infections, aging population, public awareness, rising prevalence of onychomycosis, rising disposable incomes.
The onychomycosis treatment market size is expected to see strong growth in the next few years. It will grow to $5.09 billion in 2029 at a compound annual growth rate (CAGR) of 6.7%. The growth in the forecast period can be attributed to aging population, telemedicine expansion, combination therapies, increasing access to healthcare, changing lifestyles and environmental factors, increasing prevalence of diabetes. Major trends in the forecast period include dermatological advancements, drug delivery innovations, personalized treatment plans, topical treatment advances, digital health solutions, laser therapy.
The rising incidence of fungal infections is expected to drive the growth of the onychomycosis treatment market in the coming years. Fungal infections are microbial diseases caused by various fungi, impacting different parts of the body and leading to specific conditions. Onychomycosis, or fungal nail infection, is commonly treated with oral antifungal medications or topical antifungal solutions to eradicate the underlying fungal growth and promote healthy nail regrowth. For example, in September 2023, the UK Health Security Agency, a government body in the UK, reported that there were 2,265 recorded cases of bloodstream infections (BSI) caused by yeast in England in 2022, as tracked by the SGSS. The incidence rate of BSI due to yeast increased slightly to 4 per 100,000 people, up from 3.8 per 100,000 in 2021. The three most frequently identified yeast species in blood samples were Candida albicans (40%), Nakaseomyces glabratus (previously known as Candida glabrata, 29%), and Candida parapsilosis (13%). Thus, the rising number of fungal infections is contributing to the growth of the onychomycosis treatment market.
The rising prevalence of diabetes is poised to drive the growth of the onychomycosis treatment market in the foreseeable future. Diabetes is a chronic medical condition characterized by elevated levels of blood glucose (blood sugar), increasing the likelihood of developing onychomycosis, elevating the risk of infection, and delaying the healing process. Topical and laser therapies play a crucial role in preventing complications and enhancing foot health, ultimately reducing the risk of subsequent infections. For example, in March 2021, a report from the LSU AgCenter, a Louisiana-based agricultural research center, indicated that in 2021, the United States had over 34.2 million individuals living with diabetes, and it is projected that the global population affected by diabetes will surge to 578 million by the year 2030. As a result, the growing prevalence of diabetes is a key driver behind the expansion of the onychomycosis treatment market.
Prominent companies operating in the onychomycosis treatment market are placing a strong emphasis on the development of novel products, like MOB-015, and obtaining approvals from regulatory agencies as part of their strategy to secure a competitive edge within the market. For instance, Moberg Pharma AB, a pharmaceutical company based in Sweden, which successfully obtained approval for MOB-015 from the European Union to treat mild to moderate fungal nail infections in adults. This EU approval marks a significant milestone as the first global marketing authorization for this innovative onychomycosis medication. The approval is underpinned by two Phase 3 trials supporting MOB-015, which demonstrated enhanced mycological cure rates (76% compared to up to 42% for comparators) and a substantially higher overall cure rate than the vehicle, all achieved without any occurrence of serious adverse effects. MOB-015, a topical terbinafine formulation, enables the delivery of effective terbinafine concentrations to the nail and nail bed without the associated systemic exposure risk associated with oral terbinafine treatments.
In August 2023, Karo Healthcare AB, a Swedish consumer healthcare company, completed the acquisition of Lamisil from Haleon PLC for an approximate sum of $297.86 million. This strategic move to acquire Lamisil significantly enriches Karo Healthcare's portfolio of over-the-counter (OTC) products and extends its offerings within the field of foot health. Furthermore, this transaction serves to broaden Karo's global footprint and augment its market presence in the realm of Foot Health. Lamisil, a well-known oral antifungal medication primarily utilized for the treatment of onychomycosis, was previously held by Haleon PLC, a UK-based consumer healthcare enterprise.
Major companies operating in the onychomycosis treatment market are Johnson & Johnson Services Inc., Bristol-Myers Squibb Company, Viatris Inc., Teva Pharmaceutical Industries Ltd., Hetero Drugs Ltd., Sun Pharmaceutical Industries Limited, Cadila Pharmaceuticals Ltd., Dr. Reddys Laboratories Ltd., Intas Pharmaceuticals Limited, Cipla Limited, Lupin Limited, Glenmark Pharmaceuticals Ltd., Aurobindo Pharma Limited, Alkem Laboratories Limited, Jubilant Life Sciences Ltd., Torrent Pharmaceuticals Ltd., Mankind Pharma Ltd., Biocon Limited, Emcure Pharmaceuticals Limited, Alembic Pharmaceuticals Ltd., Laurus Labs Limited, Strides Pharma Science Limited, Aarti Drugs Ltd., NATCO Pharma Ltd., Unichem Laboratories Ltd., Indoco Remedies Ltd., Apex Laboratories Pvt. Ltd., Wockhardt Ltd., Nuray Chemicals Private Limited.
North America was the largest region in the onychomycosis treatment market in 2024. Asia-Pacific is expected to be the fastest-growing region in the forecast period. The regions covered in onychomycosis treatment report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East and Africa. The countries covered in the onychomycosis treatment market report are Australia, Brazil, China, France, Germany, India, Indonesia, Japan, Russia, South Korea, UK, USA, Canada, Italy, Spain.
The onychomycosis treatment market consists of revenues earned by entities by providing combination therapy, nail debridement and Carbon dioxide ablative laser treatment. The market value includes the value of related goods sold by the service provider or included within the service offering. The onychomycosis treatment market also includes sales of griseofulvin, terbinafine, itraconazole, ketoconazole and more. Values in this market are ‘factory gate’ values, that is, the value of goods sold by the manufacturers or creators of the goods, whether to other entities (including downstream manufacturers, wholesalers, distributors and retailers) or directly to end customers. The value of goods in this market includes related services sold by the creators of the goods.
Onychomycosis treatment involves medical interventions and strategies employed to address and cure onychomycosis, a fungal infection that impacts both fingernails and toenails. The management of onychomycosis typically comprises a combination of topical and oral antifungal medications, along with preventive measures aimed at reducing the risk of recurrence.
The primary forms of onychomycosis include distal sublingual onychomycosis, white superficial onychomycosis, proximal sublingual onychomycosis, candida onychomycosis, and others. Distal sublingual onychomycosis, for instance, is a fungal nail infection that affects the underside of the nail tip, often caused by dermatophyte fungi. Treatments encompass a variety of approaches, including medications, laser therapy, and photodynamic therapy, catering to different age groups, including individuals aged 0 to 18 years, 18 to 39 years, 40 to 64 years, and those aged 65 years and above. Multiple distribution channels are involved, including hospital pharmacies, retail pharmacies, and others.
The onychomycosis treatment market research report is one of a series of new reports that provides onychomycosis treatment market statistics, including onychomycosis treatment industry global market size, regional shares, competitors with a onychomycosis treatment market share, detailed onychomycosis treatment market segments, market trends and opportunities and any further data you may need to thrive in the onychomycosis treatment industry. This onychomycosis treatment market research report delivers a complete perspective of everything you need, with an in-depth analysis of the current and future scenario of the industry.
The market value is defined as the revenues that enterprises gain from the sale of goods and/or services within the specified market and geography through sales, grants, or donations in terms of the currency (in USD unless otherwise specified).
The revenues for a specified geography are consumption values that are revenues generated by organizations in the specified geography within the market, irrespective of where they are produced. It does not include revenues from resales along the supply chain, either further along the supply chain or as part of other products.
This product will be delivered within 3-5 business days.
Table of Contents
Executive Summary
Onychomycosis Treatment Global Market Report 2025 provides strategists, marketers and senior management with the critical information they need to assess the market.This report focuses on onychomycosis treatment market which is experiencing strong growth. The report gives a guide to the trends which will be shaping the market over the next ten years and beyond.
Reasons to Purchase:
- Gain a truly global perspective with the most comprehensive report available on this market covering 15 geographies.
- Assess the impact of key macro factors such as conflict, pandemic and recovery, inflation and interest rate environment and the 2nd Trump presidency.
- Create regional and country strategies on the basis of local data and analysis.
- Identify growth segments for investment.
- Outperform competitors using forecast data and the drivers and trends shaping the market.
- Understand customers based on the latest market shares.
- Benchmark performance against key competitors.
- Suitable for supporting your internal and external presentations with reliable high quality data and analysis
- Report will be updated with the latest data and delivered to you along with an Excel data sheet for easy data extraction and analysis.
- All data from the report will also be delivered in an excel dashboard format.
Description
Where is the largest and fastest growing market for onychomycosis treatment? How does the market relate to the overall economy, demography and other similar markets? What forces will shape the market going forward? The onychomycosis treatment market global report answers all these questions and many more.The report covers market characteristics, size and growth, segmentation, regional and country breakdowns, competitive landscape, market shares, trends and strategies for this market. It traces the market’s historic and forecast market growth by geography.
- The market characteristics section of the report defines and explains the market.
- The market size section gives the market size ($b) covering both the historic growth of the market, and forecasting its development.
- The forecasts are made after considering the major factors currently impacting the market. These include:
- The forecasts are made after considering the major factors currently impacting the market. These include the Russia-Ukraine war, rising inflation, higher interest rates, and the legacy of the COVID-19 pandemic.
- Market segmentations break down the market into sub markets.
- The regional and country breakdowns section gives an analysis of the market in each geography and the size of the market by geography and compares their historic and forecast growth. It covers the growth trajectory of COVID-19 for all regions, key developed countries and major emerging markets.
- The competitive landscape chapter gives a description of the competitive nature of the market, market shares, and a description of the leading companies. Key financial deals which have shaped the market in recent years are identified.
- The trends and strategies section analyses the shape of the market as it emerges from the crisis and suggests how companies can grow as the market recovers.
Scope
Markets Covered:
1) By Type: Distal Sublingual Onychomycosis; White Superficial Onychomycosis; Proximal Sublingual Onychomycosis; Candida Onychomycosis; Other Types2) By Treatment Type: Drugs; Laser Therapy; Photodynamic Therapy
3) By Age Group: 0 To 18 Years; 18 To 39 Years; 40 To 64 Years; 65 Years And Above
4) By Distribution Channel: Hospital Pharmacies; Retail Pharmacies; Other Distribution Channels
Key Companies Mentioned: Johnson & Johnson Services Inc.; Bristol-Myers Squibb Company; Viatris Inc.; Teva Pharmaceutical Industries Ltd.; Hetero Drugs Ltd.
Countries: Australia; Brazil; China; France; Germany; India; Indonesia; Japan; Russia; South Korea; UK; USA; Canada; Italy; Spain
Regions: Asia-Pacific; Western Europe; Eastern Europe; North America; South America; Middle East; Africa
Time Series: Five years historic and ten years forecast.
Data: Ratios of market size and growth to related markets, GDP proportions, expenditure per capita.
Data Segmentation: Country and regional historic and forecast data, market share of competitors, market segments.
Sourcing and Referencing: Data and analysis throughout the report is sourced using end notes.
Delivery Format: PDF, Word and Excel Data Dashboard.
Companies Mentioned
Some of the major companies featured in this Onychomycosis Treatment market report include:- Johnson & Johnson Services Inc.
- Bristol-Myers Squibb Company
- Viatris Inc.
- Teva Pharmaceutical Industries Ltd.
- Hetero Drugs Ltd.
- Sun Pharmaceutical Industries Limited
- Cadila Pharmaceuticals Ltd.
- Dr. Reddys Laboratories Ltd.
- Intas Pharmaceuticals Limited
- Cipla Limited
- Lupin Limited
- Glenmark Pharmaceuticals Ltd.
- Aurobindo Pharma Limited
- Alkem Laboratories Limited
- Jubilant Life Sciences Ltd.
- Torrent Pharmaceuticals Ltd.
- Mankind Pharma Ltd.
- Biocon Limited
- Emcure Pharmaceuticals Limited
- Alembic Pharmaceuticals Ltd.
- Laurus Labs Limited
- Strides Pharma Science Limited
- Aarti Drugs Ltd.
- NATCO Pharma Ltd.
- Unichem Laboratories Ltd.
- Indoco Remedies Ltd.
- Apex Laboratories Pvt. Ltd.
- Wockhardt Ltd.
- Nuray Chemicals Private Limited.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 200 |
| Published | March 2025 |
| Forecast Period | 2025 - 2029 |
| Estimated Market Value ( USD | $ 3.92 Billion |
| Forecasted Market Value ( USD | $ 5.09 Billion |
| Compound Annual Growth Rate | 6.7% |
| Regions Covered | Global |
| No. of Companies Mentioned | 30 |