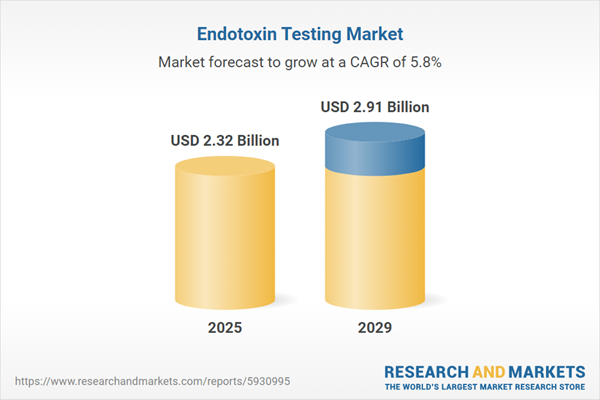
The endotoxin testing market size has grown steadily in recent years. It will grow from $2.24 billion in 2024 to $2.32 billion in 2025 at a compound annual growth rate (CAGR) of 3.6%. The growth in the historic period can be attributed to increasing demand for biologics and biosimilars, growing awareness about the importance of endotoxin testing, growing prevalence of infectious diseases.
The endotoxin testing market size is expected to see strong growth in the next few years. It will grow to $2.91 billion in 2029 at a compound annual growth rate (CAGR) of 5.8%. The growth in the forecast period can be attributed to expansion of the biopharmaceutical industry, increasing investment in research and development of endotoxin testing, global healthcare expansion. Major trends in the forecast period include innovative endotoxin detection tools, adoption of endotoxin testing service, demand for endotoxin testing services.
The growing incidence of healthcare-associated infections is poised to be a significant driver for the expansion of the endotoxin testing market. Healthcare-associated infections pertain to infections that individuals acquire while receiving medical treatment and were not present at the time of their admission to a healthcare facility. These infections often result from contaminated medical equipment or procedures, emphasizing the need for thorough testing to ensure patient safety. Endotoxin testing services play a vital role in preventing healthcare-associated infections by evaluating bacterial endotoxin levels in various medical equipment, particularly in injectable pharmaceutical products and implantable medical devices. For instance, as of December 2022, reports from the United Nations International Children's Emergency Fund (UNICEF) indicated that pneumonia was the infectious disease claiming the most children's lives, causing over 700,000 annual fatalities, which equates to approximately 2,000 children per day. Moreover, according to the World Health Organization's data from May 2022, approximately 24% of patients are affected by healthcare-associated sepsis each year, and the mortality rate for patients treated in intensive care units is as high as 52.3% annually. Consequently, the increasing prevalence of healthcare-associated infections is a major driving force behind the growth of the endotoxin testing market.
The increasing demand for biologics to conduct various tests is expected to drive the growth of the endotoxin testing market. Biologics, also referred to as biopharmaceuticals, are pharmaceuticals developed using living organisms, cells, or biological processes. Endotoxin testing is critical for biologics to ensure their safety, quality, and compliance with regulatory standards. For example, in May 2022, the European Pharmaceutical Review (EPR), a UK-based non-governmental organization, reported that biologics are projected to significantly surpass sales of innovative small molecules in the next five years, with an expected $120 billion increase in sales by 2027. This signifies a notable shift in the pharmaceutical market toward biologic products. As a result, the growing demand for biologics to conduct various tests is driving the expansion of the endotoxin testing market.
Key players in the endotoxin testing market are dedicated to embracing cutting-edge technological solutions to maintain their market standing. As an example, in August 2023, Lonza Group AG, a Switzerland-based pharmaceutical, biotechnology, and nutrition manufacturing company, introduced the Nebula absorbance reader, a novel absorbance microplate reader designed to streamline endotoxin and pyrogen testing. This instrument seamlessly integrates with Lonza's latest WinKQCL software to enhance data integrity compliance, expedite training, and reduce validation requirements. Users of WinKQCL software are no longer burdened with the need to learn new software to utilize this state-of-the-art reader, resulting in reduced training efforts. The Nebula Absorbance Reader is a forward-thinking and technologically advanced replacement reader that yields results akin to those frequently obtained with the ELx808, the industry-standard absorbance reader that Lonza no longer offers for sale. Furthermore, it is designed to be fully compatible with and meet the stringent requirements of Lonza's absorbance-based endotoxin assays, including the Lonza PYROGENT 5000 Turbidimetric and Kinetic-QCL Chromogenic Endotoxin Assays.
In January 2022, Suez Water Technologies and Solutions, a prominent water treatment company based in the United States, completed the acquisition of Sentinel Monitoring Systems Inc. for an undisclosed sum. This strategic acquisition serves to enhance Veolia Water Technologies & Solutions' analytical instrument line while unlocking new opportunities in the microbiological monitoring market. Sentinel Monitoring Systems Inc., a US-based company, specializes in the development of online bioburden monitoring tools and instrument platforms designed for endotoxin testing.
Major companies operating in the endotoxin testing market are Thermo Fisher Scientific Inc., Merck KGaA, bioMérieux SA., Eurofins Scientific SE, Lonza Group Ltd., WuXi AppTec Co. Ltd., Bio-Rad Laboratories Inc., Maravai LifeSciences Holdings Inc., Cambrex Corporation, GenScript Biotech Corporation, Charles River Laboratories International Inc., Nelson Laboratories LLC, Lifecore Biomedical Inc., Stellar Biotechnologies Inc., Biovision Inc., Associates of Cape Cod Inc., FUJIFILM Wako Pure Chemical Corporation, InvivoGen Inc., Tebu-bio Nv., MatTek Corporation, Microcoat Biotechnologie GmbH, Accelagen Inc., BioAssay Systems LLC, BioThema AB, Hycult Biotech Inc., Pacific Biolabs Inc., Xiamen Bioendo Technology Co. Ltd., Zhanjiang A&C Biological Ltd., Accugen Laboratories Inc.
North America was the largest region in the endotoxin testing market in 2024. Asia-Pacific is expected to be the fastest-growing region in the forecast period. The regions covered in endotoxin testing report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East and Africa. The countries covered in the endotoxin testing market report are Australia, Brazil, China, France, Germany, India, Indonesia, Japan, Russia, South Korea, UK, USA, Canada, Italy, Spain.
The endotoxin testing market consists of revenues earned by entities by providing contract testing services, ecologically sustainable testing and rapid testing. The market value includes the value of related goods sold by the service provider or included within the service offering. The endotoxin testing market also includes sales of injectable pharmaceutical products, endotoxin detection systems, limulus amebocyte lysate test reagents and polymerase chain reaction mycoplasma detection kits. Values in this market are ‘factory gate’ values, that is, the value of goods sold by the manufacturers or creators of the goods, whether to other entities (including downstream manufacturers, wholesalers, distributors and retailers) or directly to end customers. The value of goods in this market includes related services sold by the creators of the goods.
Endotoxin testing, also known as the bacterial endotoxins test (BET), is a laboratory-based assay designed to evaluate the levels of bacterial endotoxins in substances, with a particular focus on pharmaceuticals and medical devices. The primary goal of this testing is to ensure product safety and compliance with stringent quality standards, thereby reducing the risk of potential adverse effects on patients and users.
Endotoxin testing encompasses various methods, including LAL (limulus amebocyte lysate) tests, chromogenic tests, turbidimetric tests, gel clot tests, MAT tests, rabbit pyrogen tests, and recombinant factor C (rFC) assays. Among these, the LAL test stands out as a highly sensitive assay employed to detect bacterial endotoxins in medical and pharmaceutical products. This method leverages the clotting response of horseshoe crab blood components to identify the presence of endotoxins. The LAL test is applicable in diverse areas, such as medical devices, pharmaceuticals, packaging, and raw materials, and is utilized by various end-users, including hospitals, laboratories, and research institutes.
The endotoxin testing market research report is one of a series of new reports that provides endotoxin testing market statistics, including endotoxin testing industry global market size, regional shares, competitors with an endotoxin testing market share, detailed endotoxin testing market segments, market trends and opportunities and any further data you may need to thrive in the endotoxin testing industry. This endotoxin testing market research report delivers a complete perspective of everything you need, with an in-depth analysis of the current and future scenario of the industry.
The market value is defined as the revenues that enterprises gain from the sale of goods and/or services within the specified market and geography through sales, grants, or donations in terms of the currency (in USD, unless otherwise specified).
The revenues for a specified geography are consumption values that are revenues generated by organizations in the specified geography within the market, irrespective of where they are produced. It does not include revenues from resales along the supply chain, either further along the supply chain or as part of other products.
This product will be delivered within 3-5 business days.
Table of Contents
Executive Summary
Endotoxin Testing Global Market Report 2025 provides strategists, marketers and senior management with the critical information they need to assess the market.This report focuses on endotoxin testing market which is experiencing strong growth. The report gives a guide to the trends which will be shaping the market over the next ten years and beyond.
Reasons to Purchase:
- Gain a truly global perspective with the most comprehensive report available on this market covering 15 geographies.
- Assess the impact of key macro factors such as conflict, pandemic and recovery, inflation and interest rate environment and the 2nd Trump presidency.
- Create regional and country strategies on the basis of local data and analysis.
- Identify growth segments for investment.
- Outperform competitors using forecast data and the drivers and trends shaping the market.
- Understand customers based on the latest market shares.
- Benchmark performance against key competitors.
- Suitable for supporting your internal and external presentations with reliable high quality data and analysis
- Report will be updated with the latest data and delivered to you along with an Excel data sheet for easy data extraction and analysis.
- All data from the report will also be delivered in an excel dashboard format.
Description
Where is the largest and fastest growing market for endotoxin testing? How does the market relate to the overall economy, demography and other similar markets? What forces will shape the market going forward? The endotoxin testing market global report answers all these questions and many more.The report covers market characteristics, size and growth, segmentation, regional and country breakdowns, competitive landscape, market shares, trends and strategies for this market. It traces the market’s historic and forecast market growth by geography.
- The market characteristics section of the report defines and explains the market.
- The market size section gives the market size ($b) covering both the historic growth of the market, and forecasting its development.
- The forecasts are made after considering the major factors currently impacting the market. These include:
- The forecasts are made after considering the major factors currently impacting the market. These include the Russia-Ukraine war, rising inflation, higher interest rates, and the legacy of the COVID-19 pandemic.
- Market segmentations break down the market into sub markets.
- The regional and country breakdowns section gives an analysis of the market in each geography and the size of the market by geography and compares their historic and forecast growth. It covers the growth trajectory of COVID-19 for all regions, key developed countries and major emerging markets.
- The competitive landscape chapter gives a description of the competitive nature of the market, market shares, and a description of the leading companies. Key financial deals which have shaped the market in recent years are identified.
- The trends and strategies section analyses the shape of the market as it emerges from the crisis and suggests how companies can grow as the market recovers.
Scope
Markets Covered:
1) By Test Type: LAL (Limulus Amebocyte Lysate) Test; Chromogenic Tests; Turbidimetric Tests; Gel Clot Tests; MAT Test; Rabbit Pyrogen Test; Recombinant Factor C (rFC) Assay2) By Application: Medical Devices; Pharmaceuticals; Packaging; Raw Materials
3) By End-User: Hospitals; Laboratories; Research Institutes
Subsegments:
1) By LAL (Limulus Amebocyte Lysate) Test: Gel Clot LAL Test; Kinetic LAL Test; Endpoint LAL Test2) By Chromogenic Tests: Chromogenic LAL Test; Chromogenic Factor C Assay
3) By Turbidimetric Tests: Turbidimetric LAL Test; Turbidimetric Factor C Assay
4) By Gel Clot Tests: Gel Clot LAL Test
5) By MAT Test (Monocyte Activation Test): Direct MAT; Indirect MAT
6) By Rabbit Pyrogen Test: Standard Rabbit Pyrogen Test
7) By Recombinant Factor C (rFC) Assay: RFC Assay For Endotoxin Detection
Key Companies Mentioned: Thermo Fisher Scientific Inc.; Merck KGaA; bioMérieux SA.; Eurofins Scientific SE; Lonza Group Ltd.
Countries: Australia; Brazil; China; France; Germany; India; Indonesia; Japan; Russia; South Korea; UK; USA; Canada; Italy; Spain
Regions: Asia-Pacific; Western Europe; Eastern Europe; North America; South America; Middle East; Africa
Time Series: Five years historic and ten years forecast.
Data: Ratios of market size and growth to related markets, GDP proportions, expenditure per capita.
Data Segmentation: Country and regional historic and forecast data, market share of competitors, market segments.
Sourcing and Referencing: Data and analysis throughout the report is sourced using end notes.
Delivery Format: PDF, Word and Excel Data Dashboard.
Companies Mentioned
Some of the major companies featured in this Endotoxin Testing market report include:- Thermo Fisher Scientific Inc.
- Merck KGaA
- bioMérieux SA.
- Eurofins Scientific SE
- Lonza Group Ltd.
- WuXi AppTec Co. Ltd.
- Bio-Rad Laboratories Inc.
- Maravai LifeSciences Holdings Inc.
- Cambrex Corporation
- GenScript Biotech Corporation
- Charles River Laboratories International Inc.
- Nelson Laboratories LLC
- Lifecore Biomedical Inc.
- Stellar Biotechnologies Inc.
- Biovision Inc.
- Associates of Cape Cod Inc.
- FUJIFILM Wako Pure Chemical Corporation
- InvivoGen Inc.
- Tebu-bio Nv.
- MatTek Corporation
- Microcoat Biotechnologie GmbH
- Accelagen Inc.
- BioAssay Systems LLC
- BioThema AB
- Hycult Biotech Inc.
- Pacific Biolabs Inc.
- Xiamen Bioendo Technology Co. Ltd.
- Zhanjiang A&C Biological Ltd.
- Accugen Laboratories Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 200 |
| Published | March 2025 |
| Forecast Period | 2025 - 2029 |
| Estimated Market Value ( USD | $ 2.32 Billion |
| Forecasted Market Value ( USD | $ 2.91 Billion |
| Compound Annual Growth Rate | 5.8% |
| Regions Covered | Global |
| No. of Companies Mentioned | 30 |