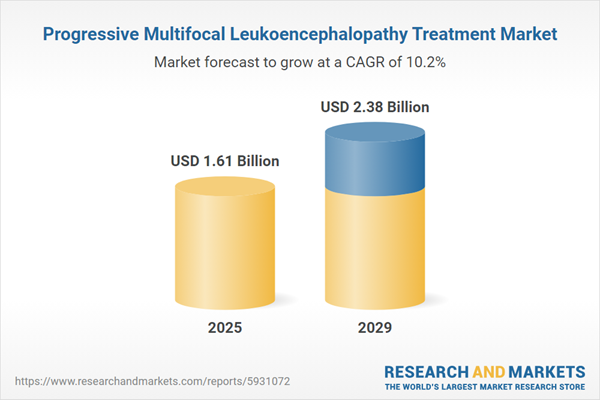
The progressive multifocal leukoencephalopathy treatment market size has grown rapidly in recent years. It will grow from $1.46 billion in 2024 to $1.61 billion in 2025 at a compound annual growth rate (CAGR) of 10.6%. The growth in the historic period can be attributed to prevalence of underlying conditions, rise in immunosuppressed patients, patient advocacy and awareness, rise of HIV/aids epidemic.
The progressive multifocal leukoencephalopathy treatment market size is expected to see rapid growth in the next few years. It will grow to $2.38 billion in 2029 at a compound annual growth rate (CAGR) of 10.2%. The growth in the forecast period can be attributed to increasing digital health and telemedicine, growing adoption of targeted therapy, improved diagnostic tools, growing awareness of progressive multifocal leukoencephalopathy (PML). Major trends in the forecast period include technological advancements in progressive multifocal leukoencephalopathy (PML) treatment, research and clinical trials, early diagnosis and screening, development of new drug therapies.
The expected increase in the prevalence of human immunodeficiency virus (HIV) infection is anticipated to be a driving force behind the expansion of the progressive multifocal leukoencephalopathy (PML) market. HIV, a retrovirus, targets the immune system, specifically CD4 cells (also known as T cells), which play a crucial role in the body's defense against infections. PML is most commonly observed in patients with HIV due to their compromised immune status, often characterized by a low CD4 cell count (below 200/L), which is the primary factor contributing to PML cases worldwide. When there is a severe immune deficiency related to HIV, the latent John Cunningham virus (JCV) archetype strain in the brain can become active, leading to the development of brain white matter lesions and PML. For instance, as of December 2022, data from the Joint United Nations Programme on HIV and AIDS (UNAIDS), a Switzerland-based United Nations initiative focused on AIDS, indicates that 630,000 (480,000-880,000) people succumbed to AIDS-related illnesses, and there were 39 million (33.1 million-45.7 million) individuals living with HIV worldwide. In 2022, an additional 1.3 million (1 million-1.7 million) people were newly diagnosed with HIV. Consequently, the increasing prevalence of HIV infection is a key factor propelling the growth of the progressive multifocal leukoencephalopathy market.
The growth of the progressive multifocal leukoencephalopathy (PML) treatment market is anticipated to be fueled by increasing research and development (R&D) activities. These activities encompass heightened efforts and investments in scientific exploration, experimentation, and innovation across various sectors. R&D is essential for advancing the treatment and management of PML, as it leads to the creation of improved therapies, earlier diagnoses, and enhanced disease management strategies. For example, in December 2023, Eurostat, a Luxembourg-based government agency, reported that the European Union allocated €352 billion ($380.4 billion) to R&D in 2022, marking a 6.34% increase from the €331 billion ($357.71 billion) invested in 2021. Thus, the rise in research and development efforts is driving growth in the PML treatment market.
Leading companies in the progressive multifocal leukoencephalopathy (PML) treatment market are increasingly dedicating their efforts to innovating their products to offer reliable solutions to their customers and to bolster their market standing. As an example, in October 2022, Cellevolve Bio, a clinical company based in the United States, obtained clearance from the United States Food and Drug Administration (FDA) for an investigational new drug (IND) application and secured orphan drug designation for CE-VST01-JC. This drug is developed to address progressive multifocal leukoencephalopathy and will undergo assessment in a global, multi-center, randomized, double-blind, placebo-controlled Phase 2 trial named ASCEND-JC. The primary objective of this trial is to compare the efficacy of CE-VST01-JC with a placebo, aiming to determine whether CE-VST01-JC can mitigate or prevent neurological progression in patients with PML. CE-VST01-JC is a T-cell therapy precisely targeting the JC virus, the causal agent of PML. This represents a significant milestone for both the PML community and Cellevolve, as ASCEND-JC stands as the largest-ever cell treatment study in the realm of PML research.
In March 2022, Cellevolve Bio, a US-based company focused on the treatment of progressive multifocal leukoencephalopathy, formed a partnership with Seattle Children’s Therapeutics. This collaboration aims to enhance the BrainChild research program, which is dedicated to developing multiplex chimeric antigen receptors (CARs) for the treatment of pediatric central nervous system (CNS) malignancies. Seattle Children’s Therapeutics is a non-profit enterprise in the United States that specializes in therapeutics development.
Major companies operating in the progressive multifocal leukoencephalopathy treatment market are Pfizer Inc., Johnson & Johnson, Roche Holding AG, Merck & Co. Inc., AbbVie Inc., Novartis AG, Bristol Myers Squibb Company, Sanofi S.A., Takeda Pharmaceutical Company Limited, Acorda Therapeutics, Eli Lilly and Company, Gilead Sciences Inc., Amgen Inc., Viatris Inc., Teva Pharmaceutical Industries Ltd., Biogen Inc., Eisai Co. Ltd., Otsuka Pharmaceutical Co. Ltd., Jazz Pharmaceuticals PLC, Ipsen Pharma, Apotex Inc., Alkermes PLC, Alnylam Pharmaceuticals Inc., Genentech Inc., Karyopharm Therapeutics Inc., ARIAD Pharmaceuticals Inc., Spectrum Pharmaceuticals, Neurimmune AG, NeoImmuneTech, Inhibikase Therapeutics Inc.
North America was the largest region in the progressive multifocal leukoencephalopathy treatment market in 2024. The regions covered in the progressive multifocal leukoencephalopathy treatment report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, the Middle East, and Africa. The countries covered in the progressive multifocal leukoencephalopathy treatment market report are Australia, Brazil, China, France, Germany, India, Indonesia, Japan, Russia, South Korea, UK, USA, Canada, Italy, and Spain.
The progressive multifocal leukoencephalopathy treatment market consists of revenues earned by entities by providing plasmapheresis, immune reconstitution therapy, physical therapy, supportive care, and consultation. The market value includes the value of related goods sold by the service provider or included within the service offering. The progressive multifocal leukoencephalopathy treatment market also includes sales of cidofovir, Brin cidofovir, and mefloquine. Values in this market are ‘factory gate’ values, that is, the value of goods sold by the manufacturers or creators of the goods, whether to other entities (including downstream manufacturers, wholesalers, distributors, and retailers) or directly to end customers. The value of goods in this market includes related services sold by the creators of the goods.
Progressive multifocal leukoencephalopathy is a rare and severe viral brain infection that primarily afflicts individuals with weakened immune systems. Treatment aims to manage symptoms, slow the disease's progression, and prevent complications. Unfortunately, there is currently no known cure for PML, and the damage caused by the virus cannot be reversed.
The main treatment approaches for progressive multifocal leukoencephalopathy include anti-retroviral therapy, antiviral or anti-John Cunningham virus (JCV) medications, and other interventions. Anti-retroviral therapy involves a combination of drugs used to manage infections caused by retroviruses, especially the human immunodeficiency virus (HIV). These therapies are employed in conditions such as HIV/AIDS, organ transplantation, multiple sclerosis, and hematologic malignancies. They can be administered through various methods, including oral and parenteral routes, and are utilized by a range of healthcare facilities, including hospitals, homecare services, specialty centers, and more.
The progressive multifocal leukoencephalopathy market research report is one of a series of new reports that provides progressive multifocal leukoencephalopathy market statistics, including progressive multifocal leukoencephalopathy industry global market size, regional shares, competitors with a progressive multifocal leukoencephalopathy market share, detailed progressive multifocal leukoencephalopathy market segments, market trends and opportunities and any further data you may need to thrive in the progressive multifocal leukoencephalopathy industry. This progressive multifocal leukoencephalopathy market research report delivers a complete perspective of everything you need, with an in-depth analysis of the current and future scenario of the industry.
The market value is defined as the revenues that enterprises gain from the sale of goods and/or services within the specified market and geography through sales, grants, or donations in terms of the currency (in USD unless otherwise specified).
The revenues for a specified geography are consumption values that are revenues generated by organizations in the specified geography within the market, irrespective of where they are produced. It does not include revenues from resales along the supply chain, either further along the supply chain or as part of other products.
This product will be delivered within 3-5 business days.
Table of Contents
Executive Summary
Progressive Multifocal Leukoencephalopathy Treatment Global Market Report 2025 provides strategists, marketers and senior management with the critical information they need to assess the market.This report focuses on progressive multifocal leukoencephalopathy treatment market which is experiencing strong growth. The report gives a guide to the trends which will be shaping the market over the next ten years and beyond.
Reasons to Purchase:
- Gain a truly global perspective with the most comprehensive report available on this market covering 15 geographies.
- Assess the impact of key macro factors such as conflict, pandemic and recovery, inflation and interest rate environment and the 2nd Trump presidency.
- Create regional and country strategies on the basis of local data and analysis.
- Identify growth segments for investment.
- Outperform competitors using forecast data and the drivers and trends shaping the market.
- Understand customers based on the latest market shares.
- Benchmark performance against key competitors.
- Suitable for supporting your internal and external presentations with reliable high quality data and analysis
- Report will be updated with the latest data and delivered to you along with an Excel data sheet for easy data extraction and analysis.
- All data from the report will also be delivered in an excel dashboard format.
Description
Where is the largest and fastest growing market for progressive multifocal leukoencephalopathy treatment? How does the market relate to the overall economy, demography and other similar markets? What forces will shape the market going forward? The progressive multifocal leukoencephalopathy treatment market global report answers all these questions and many more.The report covers market characteristics, size and growth, segmentation, regional and country breakdowns, competitive landscape, market shares, trends and strategies for this market. It traces the market’s historic and forecast market growth by geography.
- The market characteristics section of the report defines and explains the market.
- The market size section gives the market size ($b) covering both the historic growth of the market, and forecasting its development.
- The forecasts are made after considering the major factors currently impacting the market. These include:
- The forecasts are made after considering the major factors currently impacting the market. These include the Russia-Ukraine war, rising inflation, higher interest rates, and the legacy of the COVID-19 pandemic.
- Market segmentations break down the market into sub markets.
- The regional and country breakdowns section gives an analysis of the market in each geography and the size of the market by geography and compares their historic and forecast growth. It covers the growth trajectory of COVID-19 for all regions, key developed countries and major emerging markets.
- The competitive landscape chapter gives a description of the competitive nature of the market, market shares, and a description of the leading companies. Key financial deals which have shaped the market in recent years are identified.
- The trends and strategies section analyses the shape of the market as it emerges from the crisis and suggests how companies can grow as the market recovers.
Scope
Markets Covered:
1) By Treatment: Anti-retroviral Therapy; Antiviral Or Anti-John Cunningham Virus (JCV); Other Symptomatic2) By Indication: HIV Or AIDS; Organ Transplantation; Multiple Sclerosis; Hematologic Malignancies
3) By Route of Administration: Oral; Parenteral; Other Routes Of Administration
4) By End-Users: Hospitals; Homecare; Specialty Centers; Other End-Users
Subsegments:
1) By Anti-retroviral Therapy: NRTIs (Nucleoside Reverse Transcriptase Inhibitors); NNRTIs (Non-Nucleoside Reverse Transcriptase Inhibitors); PIs (Protease Inhibitors); Integrase Inhibitors2) By Antiviral Or Anti-John Cunningham Virus (JCV): Natalizumab; Fumaric Acid Esters; Other JCV-targeted Therapies
3) By Other Symptomatic Treatments: Corticosteroids; Immunomodulators; Supportive Care; Symptomatic Relief Medications
Key Companies Mentioned: Pfizer Inc.; Johnson & Johnson; Roche Holding AG; Merck & Co. Inc.; AbbVie Inc.
Countries: Australia; Brazil; China; France; Germany; India; Indonesia; Japan; Russia; South Korea; UK; USA; Canada; Italy; Spain
Regions: Asia-Pacific; Western Europe; Eastern Europe; North America; South America; Middle East; Africa
Time Series: Five years historic and ten years forecast.
Data: Ratios of market size and growth to related markets, GDP proportions, expenditure per capita.
Data Segmentation: Country and regional historic and forecast data, market share of competitors, market segments.
Sourcing and Referencing: Data and analysis throughout the report is sourced using end notes.
Delivery Format: PDF, Word and Excel Data Dashboard.
Companies Mentioned
Some of the major companies featured in this Progressive Multifocal Leukoencephalopathy Treatment market report include:- Pfizer Inc.
- Johnson & Johnson
- Roche Holding AG
- Merck & Co. Inc.
- AbbVie Inc.
- Novartis AG
- Bristol Myers Squibb Company
- Sanofi S.A.
- Takeda Pharmaceutical Company Limited
- Acorda Therapeutics
- Eli Lilly and Company
- Gilead Sciences Inc.
- Amgen Inc.
- Viatris Inc.
- Teva Pharmaceutical Industries Ltd.
- Biogen Inc.
- Eisai Co. Ltd.
- Otsuka Pharmaceutical Co. Ltd.
- Jazz Pharmaceuticals plc
- Ipsen Pharma
- Apotex Inc.
- Alkermes plc
- Alnylam Pharmaceuticals Inc.
- Genentech Inc.
- Karyopharm Therapeutics Inc.
- ARIAD Pharmaceuticals Inc.
- Spectrum Pharmaceuticals
- Neurimmune AG
- NeoImmuneTech
- Inhibikase Therapeutics Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 200 |
| Published | March 2025 |
| Forecast Period | 2025 - 2029 |
| Estimated Market Value ( USD | $ 1.61 Billion |
| Forecasted Market Value ( USD | $ 2.38 Billion |
| Compound Annual Growth Rate | 10.2% |
| Regions Covered | Global |
| No. of Companies Mentioned | 30 |