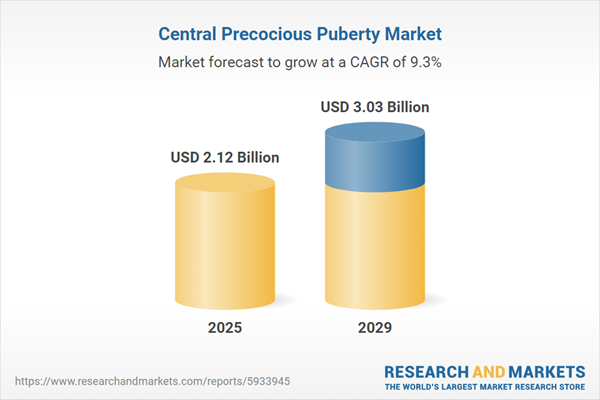
The central precocious puberty market size has grown strongly in recent years. It will grow from $1.97 billion in 2024 to $2.12 billion in 2025 at a compound annual growth rate (CAGR) of 7.8%. The growth in the historic period can be attributed to increased prevalence of central precocious puberty, improvements in treatment approaches, impact of environmental factors, increased pediatric endocrinology services, parental concerns and seeking medical attention, patient advocacy efforts.
The central precocious puberty market size is expected to see strong growth in the next few years. It will grow to $3.03 billion in 2029 at a compound annual growth rate (CAGR) of 9.3%. The growth in the forecast period can be attributed to pediatric healthcare integration, shift towards personalized medicine, parental education and awareness programs, development of longer-acting treatment options, early diagnosis and intervention. Major trends in the forecast period include long-acting formulations, patient-centric approaches, telehealth and remote monitoring, individualized treatment approaches, collaborative care models, digital health tools for monitoring.
An upsurge in research and development initiatives is anticipated to propel the expansion of the central precocious puberty market in the foreseeable future. Research and development activities encompass a spectrum of processes undertaken by organizations to innovate and improve existing knowledge, products, services, or procedures. Within the context of central precocious puberty (CPP), these efforts significantly contribute to advancing comprehension of the condition, refining diagnostic and treatment options, and elevating overall patient care. For example, Eurostat, a Luxembourg-based non-profit organization, reported an allocation of approximately $126.15 billion (€117,368 million) for research and development (R&D) by the European government in 2022, marking a 5.4% increase from the previous year's $119.73 billion (€111,393 million). Additionally, data from the Regulatory Affairs Professionals Society in January 2022 highlighted that 74% of the 37 drugs approved by the US Food and Drug Administration (FDA) in 2021 underwent expedited pathways, demonstrating a rise from the previous year's figures. This surge in research and development undertakings is a key driver of growth within the central precocious puberty market.
Rising healthcare expenditure is expected to drive the growth of the central precocious puberty (CPP) market. Healthcare expenditure encompasses the total funds allocated for healthcare goods and services, including medical equipment, hospital services, physician care, prescription drugs, and public health activities. Increased spending in healthcare benefits the CPP market by enhancing diagnosis and treatment, reducing overall healthcare costs, improving patients' quality of life, supporting the development of new treatments, and raising awareness about CPP. For example, in March 2022, the Centers for Medicare & Medicaid Services projected in its National Health Expenditure (NHE) report for 2021-2030 that national health spending would increase by an average of 5.1% annually, reaching approximately $6.8 trillion by 2030. Additionally, Medicare expenditures are expected to grow at an annual rate of 7.2%, while Medicaid spending is predicted to rise by 5.6% per year during the same period. This increase in healthcare expenditure is thus expected to fuel the growth of the CPP market.
Prominent entities within the central precocious puberty market are strategically concentrating on the development of innovative injections, such as leuprolide acetate injections, to secure a competitive advantage. Leuprolide acetate injection, a synthetic hormone emulating a natural hormone, is utilized in treating various medical conditions, including advanced prostate cancer and the premature onset of puberty. A case in point is Cipla Limited, an India-based pharmaceutical company, which introduced Leuprolide Acetate Injection Depot 22.5 mg in November 2022 for addressing central precocious puberty and advanced prostate cancer. This injection, provided in a single-dose device carrying 22.5 mg of leuprolide acetate, offers a three-month treatment duration. Comprising lyophilized microspheres in a single-dose vial, the kit includes an easy-to-use MIXJECT transfer device and a prefilled syringe containing 2 mL of 0.8% mannitol solution.
In June 2022, Eversana Life Science Services LLC, a prominent US-based life sciences company, entered into a partnership with Accord BioPharma aimed at facilitating the introduction of CAMCEVI, specifically designed for the treatment of advanced prostate cancer in adults within the United States. Although primarily intended for advanced prostate cancer, CAMCEVI (leuprolide mesylate) is also employed in addressing central precocious puberty. This synthetic hormone closely mimics a natural hormone produced in the brain and serves as a treatment for several medical conditions, including central precocious puberty. CAMCEVI stands out as the initial sterile leuprolide formulation for subcutaneous injection, packaged in a pre-filled syringe, eliminating the need for mixing before administration. Accord BioPharma, a US-based pharmaceutical company specializing in the development and marketing of treatments for various conditions, including central precocious puberty, is a key collaborator in this endeavor.
Major companies operating in the central precocious puberty market report are Cipla Limited, Cigna Group, Pfizer Inc., AbbVie Inc., Sanofi S.A., AstraZeneca PLC, Abbott Laboratories, Molina Healthcare Inc., Sun Pharmaceutical Industries Ltd., Ipsen Ltd., Ferring Pharmaceuticals Private Limited, Endo Pharmaceuticals Inc., Amneal Pharmaceuticals Inc., Piramal Enterprises Limited, Livzon Pharmaceutical Group Inc., Salvavidas Pharmaceutical Pvt. Ltd., Actiza Pharmaceutical Private Limited, The Bachem Group, Arbor Pharmaceuticals LLC, Genentech Inc., Antares Pharma Inc., Tolmar Pharmaceuticals Inc., Debiopharm Group, AmbioPharm Inc., Foresee Pharmaceuticals Co. Ltd., Manus Aktteva Biopharma LLP, Midas Pharma GmbH, LGM Pharma LLC, Varian Pharmed Group, Shenzhen JYMed Technology Co. Ltd.
North America was the largest region in the central precocious puberty market in 2024. The regions covered in the central precocious puberty market report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East, Africa. The countries covered in the central precocious puberty market report are Australia, Brazil, China, France, Germany, India, Indonesia, Japan, Russia, South Korea, UK, USA, Canada, Italy, Spain.
The central precocious puberty market consists of revenues earned by entities by providing GnRH analogue therapy, histrelin implant, and surgeries and radiations. The market value includes the value of related goods sold by the service provider or included within the service offering. The central precocious puberty market also includes sales of goserelin, gonadorelin, and buserelin, and spironolactone. Values in this market are ‘factory gate’ values, that is the value of goods sold by the manufacturers or creators of the goods, whether to other entities (including downstream manufacturers, wholesalers, distributors and retailers) or directly to end customers. The value of goods in this market includes related services sold by the creators of the goods.
Central precocious puberty is a medical condition characterized by the early onset of the normal sexual development process in both girls and boys. It occurs when the initiation of sexual development happens earlier than the typical age range, without any underlying medical problem or identifiable reason for early puberty.
The main drugs used in the treatment of central precocious puberty include leuprolide acetate, triptorelin, histrelin acetate, and nafarelin. Leuprolide acetate, a synthetic medication belonging to the gonadotropin-releasing hormone (GnRH) agonists class, is commonly used in reproductive medicine and endocrinology. These drugs can be administered through various routes, such as parenteral, subcutaneous, intramuscular, and others. They are distributed through channels such as hospital pharmacies, retail pharmacies, and online pharmacies, serving end-users such as hospitals, specialty clinics, homecare, and others.
The central precocious puberty market research report is one of a series of new reports that provides central precocious puberty market statistics, including central precocious puberty industry global market size, regional shares, competitors with a central precocious puberty market share, detailed central precocious puberty market segments, market trends and opportunities, and any further data you may need to thrive in the central precocious puberty industry. This central precocious puberty market research report delivers a complete perspective of everything you need, with an in-depth analysis of the current and future scenario of the industry.
The market value is defined as the revenues that enterprises gain from the sale of goods and/or services within the specified market and geography through sales, grants, or donations in terms of the currency (in USD, unless otherwise specified).
The revenues for a specified geography are consumption values that are revenues generated by organizations in the specified geography within the market, irrespective of where they are produced. It does not include revenues from resales along the supply chain, either further along the supply chain or as part of other products.
This product will be delivered within 3-5 business days.
Table of Contents
Executive Summary
Central Precocious Puberty Global Market Report 2025 provides strategists, marketers and senior management with the critical information they need to assess the market.This report focuses on central precocious puberty market which is experiencing strong growth. The report gives a guide to the trends which will be shaping the market over the next ten years and beyond.
Reasons to Purchase:
- Gain a truly global perspective with the most comprehensive report available on this market covering 15 geographies.
- Assess the impact of key macro factors such as conflict, pandemic and recovery, inflation and interest rate environment and the 2nd Trump presidency.
- Create regional and country strategies on the basis of local data and analysis.
- Identify growth segments for investment.
- Outperform competitors using forecast data and the drivers and trends shaping the market.
- Understand customers based on the latest market shares.
- Benchmark performance against key competitors.
- Suitable for supporting your internal and external presentations with reliable high quality data and analysis
- Report will be updated with the latest data and delivered to you along with an Excel data sheet for easy data extraction and analysis.
- All data from the report will also be delivered in an excel dashboard format.
Description
Where is the largest and fastest growing market for central precocious puberty? How does the market relate to the overall economy, demography and other similar markets? What forces will shape the market going forward? The central precocious puberty market global report answers all these questions and many more.The report covers market characteristics, size and growth, segmentation, regional and country breakdowns, competitive landscape, market shares, trends and strategies for this market. It traces the market’s historic and forecast market growth by geography.
- The market characteristics section of the report defines and explains the market.
- The market size section gives the market size ($b) covering both the historic growth of the market, and forecasting its development.
- The forecasts are made after considering the major factors currently impacting the market. These include:
- The forecasts are made after considering the major factors currently impacting the market. These include the Russia-Ukraine war, rising inflation, higher interest rates, and the legacy of the COVID-19 pandemic.
- Market segmentations break down the market into sub markets.
- The regional and country breakdowns section gives an analysis of the market in each geography and the size of the market by geography and compares their historic and forecast growth. It covers the growth trajectory of COVID-19 for all regions, key developed countries and major emerging markets.
- The competitive landscape chapter gives a description of the competitive nature of the market, market shares, and a description of the leading companies. Key financial deals which have shaped the market in recent years are identified.
- The trends and strategies section analyses the shape of the market as it emerges from the crisis and suggests how companies can grow as the market recovers.
Scope
Markets Covered:
1) By Drug: Leuprolide Acetate; Triptorelin; Histrelin Acetate; Nafarelin2) By Route Of Administration: Parenteral; Subcutaneous; Intramuscular; Other Routes Of Administrations
3) By Distribution Channel: Hospital Pharmacy; Retail Pharmacy; Online Pharmacy
4) By End-Users: Hospitals; Specialty Clinics; Homecare; Other End-Users
Subsegments:
1) By Leuprolide Acetate: Injectable Formulations; Depot Formulations2) By Triptorelin: Injectable Formulations; Long-acting Formulations
3) By Histrelin Acetate: Implant Formulations; Injectable Formulations
4) By Nafarelin: Nasal Spray Formulations; Injectable Formulations
Key Companies Mentioned: Cipla Limited; Cigna Group; Pfizer Inc.; AbbVie Inc.; Sanofi S.A.
Countries: Australia; Brazil; China; France; Germany; India; Indonesia; Japan; Russia; South Korea; UK; USA; Canada; Italy; Spain
Regions: Asia-Pacific; Western Europe; Eastern Europe; North America; South America; Middle East; Africa
Time Series: Five years historic and ten years forecast.
Data: Ratios of market size and growth to related markets, GDP proportions, expenditure per capita.
Data Segmentation: Country and regional historic and forecast data, market share of competitors, market segments.
Sourcing and Referencing: Data and analysis throughout the report is sourced using end notes.
Delivery Format: PDF, Word and Excel Data Dashboard.
Companies Mentioned
Some of the major companies featured in this Central Precocious Puberty market report include:- Cipla Limited
- Cigna Group
- Pfizer Inc.
- AbbVie Inc.
- Sanofi S.A.
- AstraZeneca PLC
- Abbott Laboratories
- Molina Healthcare Inc.
- Sun Pharmaceutical Industries Ltd.
- Ipsen Ltd.
- Ferring Pharmaceuticals Private Limited
- Endo Pharmaceuticals Inc.
- Amneal Pharmaceuticals Inc.
- Piramal Enterprises Limited
- Livzon Pharmaceutical Group Inc.
- Salvavidas Pharmaceutical Pvt. Ltd.
- Actiza Pharmaceutical Private Limited
- The Bachem Group
- Arbor Pharmaceuticals LLC
- Genentech Inc.
- Antares Pharma Inc.
- Tolmar Pharmaceuticals Inc.
- Debiopharm Group
- AmbioPharm Inc.
- Foresee Pharmaceuticals Co. Ltd.
- Manus Aktteva Biopharma LLP
- Midas Pharma GmbH
- LGM Pharma LLC
- Varian Pharmed Group
- Shenzhen JYMed Technology Co. Ltd.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 200 |
| Published | March 2025 |
| Forecast Period | 2025 - 2029 |
| Estimated Market Value ( USD | $ 2.12 Billion |
| Forecasted Market Value ( USD | $ 3.03 Billion |
| Compound Annual Growth Rate | 9.3% |
| Regions Covered | Global |
| No. of Companies Mentioned | 31 |