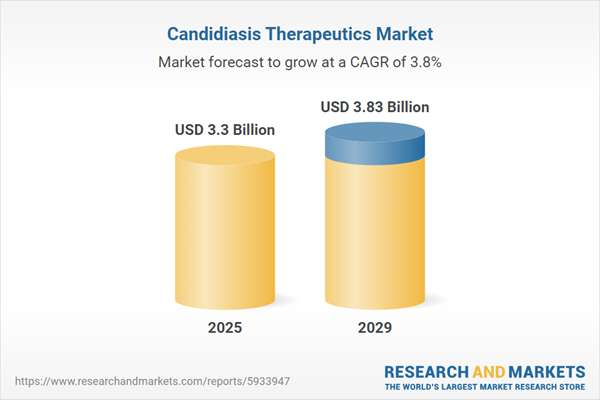
The candidiasis therapeutics market size has grown steadily in recent years. It will grow from $3.19 billion in 2024 to $3.3 billion in 2025 at a compound annual growth rate (CAGR) of 3.4%. The growth in the historic period can be attributed to increased incidence of candidiasis, antibiotic use and resistance, hospital-acquired infections, shift in fungal resistance patterns, awareness and education programs.
The candidiasis therapeutics market size is expected to see steady growth in the next few years. It will grow to $3.83 billion in 2029 at a compound annual growth rate (CAGR) of 3.8%. The growth in the forecast period can be attributed to precision medicine approaches, global antifungal stewardship programs, increasing prevalence of candida auris, global efforts against antifungal resistance. Major trends in the forecast period include advancements in drug delivery technologies, telemedicine and remote patient monitoring, patient-centric care, combination therapies, collaborative research initiatives.
The increasing prevalence of fungal infections is expected to drive the growth of the candidiasis therapeutics market. Fungal infections, which affect the skin, mucous membranes, and internal organs, are more common in individuals with weakened immune systems. This growing incidence of infections, particularly candidiasis, creates opportunities for manufacturers to develop and provide specialized, effective antifungal drugs that target the underlying pathogens. These drugs aim to alleviate symptoms, prevent complications, promote recovery, reduce the spread of Candida infections in healthcare settings, and improve patient outcomes. For instance, in January 2024, the University of Minnesota, a U.S.-based research institution, reported that fungal infections cause more than 3.75 million deaths annually, with about 2.55 million deaths directly linked to fungal diseases. As a result, the rising number of fungal infections is expected to propel the candidiasis therapeutics market.
The increasing number of clinical trials is expected to further fuel the growth trajectory of the candidiasis therapeutics market. Clinical trials serve as pivotal research studies aimed at assessing the safety and efficacy of novel medical treatments, interventions, drugs, or devices in human subjects. Within the domain of candidiasis therapeutics, these trials play a crucial role by offering a systematic and stringent evaluation platform for testing the safety and effectiveness of potential treatments. As reported by ClinicalTrials.gov in May 2023, the number of registered clinical trials surged to around 437,533 in 2023, compared to 399,499 registered studies in 2022 across all 50 states of the United States and spanning 221 countries. This increase underscores the rising momentum and significance of clinical trials in shaping the landscape of candidiasis therapeutics. Consequently, the burgeoning number of clinical trials emerges as a key factor driving the growth of the candidiasis therapeutics market.
Leading companies operating within the candidiasis therapeutics market are dedicatedly engaged in developing innovative drugs, such as BREXAFEMME (ibrexafungerp tablets), aimed at mitigating the occurrence of recurrent vulvovaginal candidiasis (RVVC) in adult women. BREXAFEMME (ibrexafungerp tablets) constitutes an oral, non-azole medication designed for the treatment of vulvovaginal candidiasis (VVC) and reducing the incidence of recurrent vulvovaginal candidiasis (RVVC), commonly known as chronic yeast infections. For instance, in December 2022, Scynexis Inc., a prominent US-based pharmaceutical company, secured approval from the US Food and Drug Administration (FDA) for BREXAFEMME (ibrexafungerp tablets). The medication targets Candida albicans, the fungus responsible for VVC, by obstructing its growth. Notably, BREXAFEMME is deemed a safe and effective treatment option for vulvovaginal candidiasis (VVC), available solely through prescription, with a recommended dosage of one 150-mg capsule taken orally once daily for a single day. This innovative drug stands as a promising advancement in addressing candidiasis, offering a viable treatment solution while navigating potential concerns related to adverse drug effects.
In May 2023, GSK plc, a prominent UK-based pharmaceutical company, finalized the acquisition of Licensing rights for ibrexafungerp from Scynexism Inc. in a transaction amounting to $90 million. This strategic acquisition grants GSK exclusive rights to further the development and commercialization of ibrexafungerp drugs, a groundbreaking antifungal medication designed for the treatment of vulvovaginal candidiasis (VVC). The acquisition is aimed at expediting the advancement and market availability of ibrexafungerp. Scynexism Inc., a notable US-based pharmaceutical company specializing in therapeutic solutions for fungal diseases, including candidiasis, initially developed this innovative medication.
Major companies operating in the candidiasis therapeutics market report are Pfizer Inc., Biocon Limited, Torrent Pharmaceuticals Ltd., F. Hoffmann-La Roche Ltd., Merck & Co. Inc., AbbVie Inc., Bayer AG, Novartis AG, Sanofi S.A., AstraZeneca plc., GSK plc., Amgen Inc., Teva Pharmaceutical Industries Ltd., Astellas Pharma Inc., Cipla Inc., Bruker Corporation, Lupin Limited, Galapagos NV, Basilea Pharmaceutica Ltd., WOCKHARDT Limited, Pacgen Life Science Corporation, Cidara Therapeutics Inc., Ablynx NV, Amplyx Pharmaceuticals Inc., NovaDigm Therapeutics Inc., SCYNEXIS Inc., Medivir AB, Mycovia Pharmaceuticals Inc., NovaBiotics Inc., Matinas BioPharma Holdings Inc.
North America was the largest region in the candidiasis therapeutics market in 2024. Asia-Pacific is expected to be the fastest-growing region in the forecast period. The regions covered in the candidiasis therapeutics market report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East, Africa. The countries covered in the candidiasis therapeutics market report are Australia, Brazil, China, France, Germany, India, Indonesia, Japan, Russia, South Korea, UK, USA, Canada, Italy, Spain.
The candidiasis therapeutics market consists of revenues earned by entities by providing services such as medication services, diagnostic tests, consultation services, and medical monitoring services. The market value includes the value of related goods sold by the service provider or included within the service offering. The candidiasis therapeutics market also includes sales of topical creams and ointments, oral medications, medical devices, probiotics, nutritional supplements and antifungal medications that are used in providing these services. Values in this market are ‘factory gate’ values, that is the value of goods sold by the manufacturers or creators of the goods, whether to other entities (including downstream manufacturers, wholesalers, distributors and retailers) or directly to end customers. The value of goods in this market includes related services sold by the creators of the goods.
Candidiasis, a fungal infection caused by the overgrowth of Candida species, commonly affects areas such as the mouth, throat, gut, and genital regions. The primary methods of treating candidiasis involve antifungal medications, which can be either topical (applied to the affected area) or oral (ingested), depending on the severity and location of the infection.
Candidiasis therapeutics encompass various treatment approaches, including medication, surgery, and other interventions. Medication involves the use of pharmaceutical drugs to manage or alleviate the condition. The treatments target specific anatomical sites such as oral candidiasis, vulvovaginal candidiasis, cutaneous candidiasis, invasive candidiasis, and systemic candidiasis. These medications can be administered through various routes, including injectable, oral, and others, catering to both adult and pediatric patients. The end-users for these treatments include hospitals, homecare settings, specialty clinics, and other healthcare facilities.
The candidiasis therapeutics market research report is one of a series of new reports that provides candidiasis therapeutics market statistics, including candidiasis therapeutics industry global market size, regional shares, competitors with a candidiasis therapeutics market share, detailed candidiasis therapeutics market segments, market trends and opportunities, and any further data you may need to thrive in the candidiasis therapeutics industry. This candidiasis therapeutics market research report delivers a complete perspective of everything you need, with an in-depth analysis of the current and future scenario of the industry.
The market value is defined as the revenues that enterprises gain from the sale of goods and/or services within the specified market and geography through sales, grants, or donations in terms of the currency (in USD, unless otherwise specified).
The revenues for a specified geography are consumption values that are revenues generated by organizations in the specified geography within the market, irrespective of where they are produced. It does not include revenues from resales along the supply chain, either further along the supply chain or as part of other products.
This product will be delivered within 3-5 business days.
Table of Contents
Executive Summary
Candidiasis Therapeutics Global Market Report 2025 provides strategists, marketers and senior management with the critical information they need to assess the market.This report focuses on candidiasis therapeutics market which is experiencing strong growth. The report gives a guide to the trends which will be shaping the market over the next ten years and beyond.
Reasons to Purchase:
- Gain a truly global perspective with the most comprehensive report available on this market covering 15 geographies.
- Assess the impact of key macro factors such as conflict, pandemic and recovery, inflation and interest rate environment and the 2nd Trump presidency.
- Create regional and country strategies on the basis of local data and analysis.
- Identify growth segments for investment.
- Outperform competitors using forecast data and the drivers and trends shaping the market.
- Understand customers based on the latest market shares.
- Benchmark performance against key competitors.
- Suitable for supporting your internal and external presentations with reliable high quality data and analysis
- Report will be updated with the latest data and delivered to you along with an Excel data sheet for easy data extraction and analysis.
- All data from the report will also be delivered in an excel dashboard format.
Description
Where is the largest and fastest growing market for candidiasis therapeutics? How does the market relate to the overall economy, demography and other similar markets? What forces will shape the market going forward? The candidiasis therapeutics market global report answers all these questions and many more.The report covers market characteristics, size and growth, segmentation, regional and country breakdowns, competitive landscape, market shares, trends and strategies for this market. It traces the market’s historic and forecast market growth by geography.
- The market characteristics section of the report defines and explains the market.
- The market size section gives the market size ($b) covering both the historic growth of the market, and forecasting its development.
- The forecasts are made after considering the major factors currently impacting the market. These include:
- The forecasts are made after considering the major factors currently impacting the market. These include the Russia-Ukraine war, rising inflation, higher interest rates, and the legacy of the COVID-19 pandemic.
- Market segmentations break down the market into sub markets.
- The regional and country breakdowns section gives an analysis of the market in each geography and the size of the market by geography and compares their historic and forecast growth. It covers the growth trajectory of COVID-19 for all regions, key developed countries and major emerging markets.
- The competitive landscape chapter gives a description of the competitive nature of the market, market shares, and a description of the leading companies. Key financial deals which have shaped the market in recent years are identified.
- The trends and strategies section analyses the shape of the market as it emerges from the crisis and suggests how companies can grow as the market recovers.
Scope
Markets Covered:
1) By Treatment Type: Medication; Surgery; Other Treatments2) By Anatomy Type: Oral Candidiasis; Vulvovaginal Candidiasis; Cutaneous Candidiasis; Invasive Candidiasis; Systemic Candidiasis
3) By Mode Of Administration: Injectable; Oral; Other Mode Of Administrations
4) By Patient Population: Adults; Pediatrics
5) By End User: Hospitals; Homecare; Specialty Clinics; Other End Users
Subsegments:
1) By Medication: Antifungal Drugs; Topical Treatments; Systemic Treatments2) By Surgery: Surgical Debridement; Organ Removal
3) By Other Treatments: Probiotics; Home Remedies; Lifestyle Modifications
Key Companies Mentioned: Pfizer Inc.; Biocon Limited; Torrent Pharmaceuticals Ltd.; F. Hoffmann-La Roche Ltd.; Merck & Co. Inc.
Countries: Australia; Brazil; China; France; Germany; India; Indonesia; Japan; Russia; South Korea; UK; USA; Canada; Italy; Spain
Regions: Asia-Pacific; Western Europe; Eastern Europe; North America; South America; Middle East; Africa
Time Series: Five years historic and ten years forecast.
Data: Ratios of market size and growth to related markets, GDP proportions, expenditure per capita.
Data Segmentation: Country and regional historic and forecast data, market share of competitors, market segments.
Sourcing and Referencing: Data and analysis throughout the report is sourced using end notes.
Delivery Format: PDF, Word and Excel Data Dashboard.
Companies Mentioned
Some of the major companies featured in this Candidiasis Therapeutics market report include:- Pfizer Inc.
- Biocon Limited
- Torrent Pharmaceuticals Ltd.
- F. Hoffmann-La Roche Ltd.
- Merck & Co. Inc.
- AbbVie Inc.
- Bayer AG
- Novartis AG
- Sanofi S.A.
- AstraZeneca plc.
- GSK plc.
- Amgen Inc.
- Teva Pharmaceutical Industries Ltd.
- Astellas Pharma Inc.
- Cipla Inc.
- Bruker Corporation
- Lupin Limited
- Galapagos NV
- Basilea Pharmaceutica Ltd.
- WOCKHARDT Limited
- Pacgen Life Science Corporation
- Cidara Therapeutics Inc.
- Ablynx NV
- Amplyx Pharmaceuticals Inc.
- NovaDigm Therapeutics Inc.
- SCYNEXIS Inc.
- Medivir AB
- Mycovia Pharmaceuticals Inc.
- NovaBiotics Inc.
- Matinas BioPharma Holdings Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 200 |
| Published | March 2025 |
| Forecast Period | 2025 - 2029 |
| Estimated Market Value ( USD | $ 3.3 Billion |
| Forecasted Market Value ( USD | $ 3.83 Billion |
| Compound Annual Growth Rate | 3.8% |
| Regions Covered | Global |
| No. of Companies Mentioned | 31 |