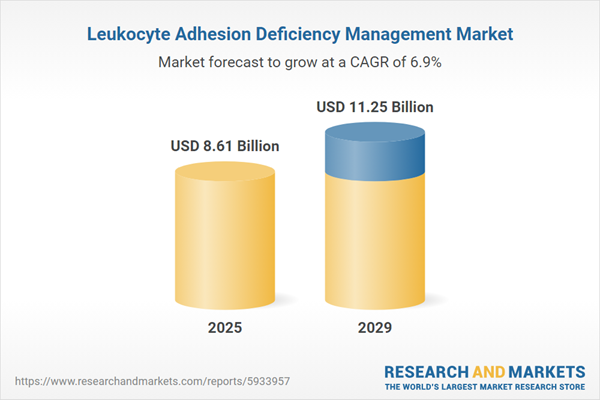
The leukocyte adhesion deficiency management market size has grown strongly in recent years. It will grow from $8.07 billion in 2024 to $8.61 billion in 2025 at a compound annual growth rate (CAGR) of 6.7%. The growth in the historic period can be attributed to increased awareness and education, genetic testing advancements, orphan drug designations, global health policies, patient advocacy groups, pediatric healthcare advancements.
The leukocyte adhesion deficiency management market size is expected to see strong growth in the next few years. It will grow to $11.25 billion in 2029 at a compound annual growth rate (CAGR) of 6.9%. The growth in the forecast period can be attributed to precision medicine approaches, emergence of novel therapeutics, expanded genetic testing accessibility, patient-centric drug development, advancements in immunomodulatory therapies. Major trends in the forecast period include stem cell transplantation innovations, integration of supportive therapies, telemedicine for remote consultations, collaboration and research networks, integration of biomarker technologies.
The rising incidence of primary immunodeficiency (PID) is anticipated to drive the expansion of the leukocyte adhesion deficiency management market. PID encompasses a range of inherited disorders that compromise the immune system's functionality, rendering individuals more vulnerable to infections and diseases due to deficiencies in their immune responses. Managing leukocyte adhesion deficiency can significantly benefit those with primary immunodeficiency, potentially enhancing the understanding of immune system disorders and fostering the development of improved diagnostic techniques and therapies for these conditions. For example, statistics gathered during World Primary Immunodeficiency (WPI) Awareness Week 2023 by the Peace Hospital indicated that an estimated 60 lakh individuals worldwide are affected by PI, with 70% to 90% of cases remaining undiagnosed. Hence, the rising prevalence of primary immunodeficiency is set to propel the growth of the leukocyte adhesion deficiency management market.
The increasing prevalence of spinal cord injuries is expected to drive the growth of the leukocyte adhesion deficiency management market. Spinal cord injury, resulting from damage to any part of the spinal cord or associated nerves, often leads to complications such as autonomic dysfunction, including conditions such as hyperhidrosis. This issue is linked to heightened sympathetic activity in the spinal cord segment located just below the injury site. As indicated by the National Spinal Cord Injury Statistical Center (NSCISC) in March 2022, the estimated annual incidence of traumatic spinal cord injury (SCI) in the United States stands at approximately 54 cases per one million people, equating to about 18,000 new SCI cases annually. Moreover, the estimated count of individuals living with SCI in the United States ranges between 253,000 to 378,000, totaling around 299,000. Hence, the increasing prevalence of spinal cord injuries contributes to the growth of the leukocyte adhesion deficiency management market.
Key players in the leukocyte adhesion deficiency (LAD) management market are advancing lentiviral vector-based gene therapy technology to improve targeted treatments and enhance patient outcomes. This approach delivers corrective genes directly into cells, potentially restoring immune function, providing a more precise solution for managing LAD. For example, in February 2024, Rocket Pharmaceuticals, a US-based biotechnology firm, introduced Kresladi (marnetegragene autotemcel) as a treatment for severe leukocyte adhesion deficiency-I (LAD-I). In a Phase 1/2 clinical trial, Kresladi demonstrated a 100% survival rate at 12 months, significantly reducing infections and improving patient symptoms. This innovation presents a promising new option for LAD treatment, addressing critical unmet medical needs in the market.
In February 2023, the Jeffrey Modell Foundation (JMF), a non-profit organization dedicated to primary immunodeficiency, entered into a collaboration with Veritas Intercontinental, a healthcare solutions company based in Spain. The collaboration's primary objective is to advance the field of genome and exome sequencing in the context of immunological disorders. By leveraging global expertise and resources, the partnership seeks to identify gene abnormalities associated with primary immunodeficiency conditions, including conditions such as leukocyte adhesion deficiency. This joint effort aims to significantly improve the accuracy of diagnosis and treatment protocols by enhancing the understanding of genetic factors contributing to these immunological disorders.
Major companies operating in the leukocyte adhesion deficiency management market report are F. Hoffmann-La Roche Ltd., Novartis AG, GlaxoSmithKline PLC, Gilead Sciences Inc., Teva Pharmaceuticals Industries Limited, CSL Behring LLC, Vertex Pharmaceuticals Inc., Grifols International S.A., Aurobindo Pharma Ltd., BioMarin Pharmaceutical Inc., Cadila Healthcare Ltd., Ipca Laboratories Ltd., Sana Biotechnology Inc., Rocket Pharmaceuticals Inc., PT Sanbe Farma, Magenta Therapeutics Inc., Avalo Therapeutics Inc., Aspen Neuroscience Inc., Sandoz International GmbH, Orpha Labs Inc., Swiss Pharm Holding AG, Rubius Therapeutics Inc., Enochian Biosciences Inc., Sigma-Aldrich LLC, Bluebird Bio Inc.
North America was the largest region in the leukocyte adhesion deficiency management market in 2024. Asia-Pacific is expected to be the fastest-growing region in the forecast period. The regions covered in the leukocyte adhesion deficiency management market report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East, Africa. The countries covered in the leukocyte adhesion deficiency management market report are Australia, Brazil, China, France, Germany, India, Indonesia, Japan, Russia, South Korea, UK, USA, Canada, Italy, Spain.
The leukocyte adhesion deficiency management market consists of revenues earned by entities by providing services such as gene therapy, granulocyte transfusions, nutrition, and supportive care. The market value includes the value of related goods sold by the service provider or included within the service offering. The leukocyte adhesion deficiency management market also includes sales of antibiotics such as trimethoprim and sulfamethoxazole. Values in this market are ‘factory gate’ values, that is the value of goods sold by the manufacturers or creators of the goods, whether to other entities (including downstream manufacturers, wholesalers, distributors and retailers) or directly to end customers. The value of goods in this market includes related services sold by the creators of the goods.
Leukocyte Adhesion Deficiency (LAD) is a rare genetic disorder that impacts the immune system's ability to combat infections, specifically by affecting the adhesion of white blood cells, particularly neutrophils. These cells are crucial for defending the body against bacteria and fungi. Managing leukocyte adhesion deficiency requires a comprehensive approach, focusing on preventing and treating infections while addressing the underlying immune system dysfunction.
The primary treatments for managing leukocyte adhesion deficiency include hematopoietic stem cell transplantation, recombinant human interferon-gamma treatment, prophylactic immunoglobulin therapy, antimicrobial therapy, prophylactic therapy, fucose supplementation, monoclonal antibodies, and coagulation factors. Hematopoietic stem cell transplantation involves transplanting stem cells from the bone marrow or blood to replace damaged or diseased blood-forming cells. Treatment and diagnostic services, such as blood tests, genetic testing, biopsies, are provided by various end users, including hospitals and specialty clinics.
The leukocyte adhesion deficiency management market research report is one of a series of new reports that provides leukocyte adhesion deficiency management market statistics, including leukocyte adhesion deficiency management industry global market size, regional shares, competitors with a leukocyte adhesion deficiency management market share, detailed leukocyte adhesion deficiency management market segments, market trends and opportunities, and any further data you may need to thrive in the leukocyte adhesion deficiency management industry. This leukocyte adhesion deficiency management market research report delivers a complete perspective of everything you need, with an in-depth analysis of the current and future scenario of the industry.
The market value is defined as the revenues that enterprises gain from the sale of goods and/or services within the specified market and geography through sales, grants, or donations in terms of the currency (in USD, unless otherwise specified).
The revenues for a specified geography are consumption values that are revenues generated by organizations in the specified geography within the market, irrespective of where they are produced. It does not include revenues from resales along the supply chain, either further along the supply chain or as part of other products.
This product will be delivered within 3-5 business days.
Table of Contents
Executive Summary
Leukocyte Adhesion Deficiency Management Global Market Report 2025 provides strategists, marketers and senior management with the critical information they need to assess the market.This report focuses on leukocyte adhesion deficiency management market which is experiencing strong growth. The report gives a guide to the trends which will be shaping the market over the next ten years and beyond.
Reasons to Purchase:
- Gain a truly global perspective with the most comprehensive report available on this market covering 15 geographies.
- Assess the impact of key macro factors such as conflict, pandemic and recovery, inflation and interest rate environment and the 2nd Trump presidency.
- Create regional and country strategies on the basis of local data and analysis.
- Identify growth segments for investment.
- Outperform competitors using forecast data and the drivers and trends shaping the market.
- Understand customers based on the latest market shares.
- Benchmark performance against key competitors.
- Suitable for supporting your internal and external presentations with reliable high quality data and analysis
- Report will be updated with the latest data and delivered to you along with an Excel data sheet for easy data extraction and analysis.
- All data from the report will also be delivered in an excel dashboard format.
Description
Where is the largest and fastest growing market for leukocyte adhesion deficiency management? How does the market relate to the overall economy, demography and other similar markets? What forces will shape the market going forward? The leukocyte adhesion deficiency management market global report answers all these questions and many more.The report covers market characteristics, size and growth, segmentation, regional and country breakdowns, competitive landscape, market shares, trends and strategies for this market. It traces the market’s historic and forecast market growth by geography.
- The market characteristics section of the report defines and explains the market.
- The market size section gives the market size ($b) covering both the historic growth of the market, and forecasting its development.
- The forecasts are made after considering the major factors currently impacting the market. These include:
- The forecasts are made after considering the major factors currently impacting the market. These include the Russia-Ukraine war, rising inflation, higher interest rates, and the legacy of the COVID-19 pandemic.
- Market segmentations break down the market into sub markets.
- The regional and country breakdowns section gives an analysis of the market in each geography and the size of the market by geography and compares their historic and forecast growth. It covers the growth trajectory of COVID-19 for all regions, key developed countries and major emerging markets.
- The competitive landscape chapter gives a description of the competitive nature of the market, market shares, and a description of the leading companies. Key financial deals which have shaped the market in recent years are identified.
- The trends and strategies section analyses the shape of the market as it emerges from the crisis and suggests how companies can grow as the market recovers.
Scope
Markets Covered:
1) By Treatment: Hematopoietic Stem Cell Transplantation; Recombinant Human Interferon-Gamma Treatment; Prophylactic Immunoglobulin Therapy; Antimicrobial Therapy; Prophylactic Therapy; Fucose Supplementation; Monoclonal Antibodies; Coagulation Factors2) By Diagnosis: Blood Test; Genetic Testing; Biopsy; Other Types
3) By End-Users: Hospitals; Specialty Clinics; Other End-Users
Subsegments:
1) By Hematopoietic Stem Cell Transplantation: Related Donor Transplant; Unrelated Donor Transplant2) By Recombinant Human Interferon-Gamma Treatment: Standard Dosing; Customized Dosing
3) By Prophylactic Immunoglobulin Therapy: Intravenous Immunoglobulin (IVIG); Subcutaneous Immunoglobulin (SCIG)
4) By Antimicrobial Therapy: Antibiotics; Antifungal Treatments
5) By Prophylactic Therapy: Vaccination Strategies; Infection Prevention Protocols
6) By Fucose Supplementation: Oral Supplements; Intravenous Supplements
7) By Monoclonal Antibodies: Specific Target Monoclonal Antibodies; Combination Therapy Monoclonal Antibodies
8) By Coagulation Factors: Factor Replacement Therapies; Novel Coagulation Agents
Key Companies Mentioned: F. Hoffmann-La Roche Ltd.; Novartis AG; GlaxoSmithKline Plc; Gilead Sciences Inc; Teva Pharmaceuticals Industries Limited
Countries: Australia; Brazil; China; France; Germany; India; Indonesia; Japan; Russia; South Korea; UK; USA; Canada; Italy; Spain
Regions: Asia-Pacific; Western Europe; Eastern Europe; North America; South America; Middle East; Africa
Time Series: Five years historic and ten years forecast.
Data: Ratios of market size and growth to related markets, GDP proportions, expenditure per capita.
Data Segmentation: Country and regional historic and forecast data, market share of competitors, market segments.
Sourcing and Referencing: Data and analysis throughout the report is sourced using end notes.
Delivery Format: PDF, Word and Excel Data Dashboard.
Companies Mentioned
Some of the major companies featured in this Leukocyte Adhesion Deficiency Management market report include:- F. Hoffmann-La Roche Ltd.
- Novartis AG
- GlaxoSmithKline Plc
- Gilead Sciences Inc
- Teva Pharmaceuticals Industries Limited
- CSL Behring LLC
- Vertex Pharmaceuticals Inc.
- Grifols International S.A.
- Aurobindo Pharma Ltd.
- BioMarin Pharmaceutical Inc.
- Cadila Healthcare Ltd.
- Ipca Laboratories Ltd
- Sana Biotechnology Inc.
- Rocket Pharmaceuticals Inc.
- PT Sanbe Farma
- Magenta Therapeutics Inc
- Avalo Therapeutics Inc.
- Aspen Neuroscience Inc.
- Sandoz International GmbH
- Orpha Labs Inc.
- Swiss Pharm Holding AG
- Rubius Therapeutics Inc.
- Enochian Biosciences Inc.
- Sigma-Aldrich LLC
- Bluebird Bio Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 200 |
| Published | March 2025 |
| Forecast Period | 2025 - 2029 |
| Estimated Market Value ( USD | $ 8.61 Billion |
| Forecasted Market Value ( USD | $ 11.25 Billion |
| Compound Annual Growth Rate | 6.9% |
| Regions Covered | Global |
| No. of Companies Mentioned | 25 |