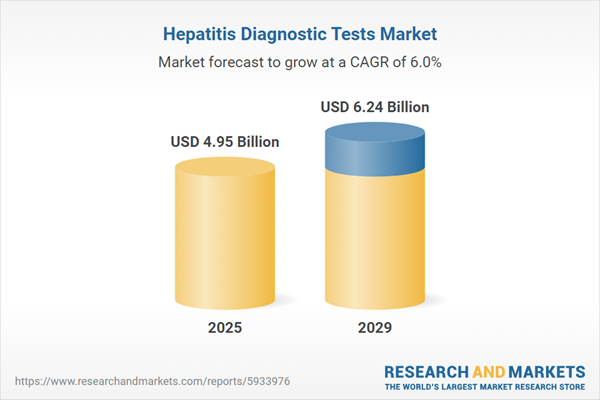
The hepatitis diagnostic tests market size has grown strongly in recent years. It will grow from $4.64 billion in 2024 to $4.95 billion in 2025 at a compound annual growth rate (CAGR) of 6.5%. The growth in the historic period can be attributed to globalization of healthcare, government initiatives, high disease burden, increased blood safety measures, public health campaigns.
The hepatitis diagnostic tests market size is expected to see strong growth in the next few years. It will grow to $6.24 billion in 2029 at a compound annual growth rate (CAGR) of 6%. The growth in the forecast period can be attributed to global health security concerns, population growth and urbanization, tailored testing strategies, emphasis on elimination programs, increasing healthcare spending. Major trends in the forecast period include advancements in testing technologies, multiplex testing for different hepatitis viruses, integration of molecular diagnostics, digital health solutions, strategic collaborations and partnerships.
The increasing number of blood transfusions and donations is expected to drive growth in the hepatitis diagnostic test market. Blood transfusions and donations are connected processes in which individuals voluntarily provide blood, which is then collected, tested, and administered to patients in need of additional blood components due to medical conditions, surgeries, accidents, or illnesses. Hepatitis diagnostic tests are essential for thoroughly screening blood before donation or transfusion, ensuring patient safety. For instance, in August 2023, the Royal College of Pathologists, a UK-based organization, reported that blood transfusion is the most commonly performed procedure for hospitalized patients, with nearly 2 million blood components issued and transfused annually in England, costing over £300 million ($390.69 million). Thus, the growing number of blood transfusions and donations supports the expansion of the hepatitis diagnostic test market.
The surge in hepatitis cases globally is another key factor propelling the growth of the hepatitis diagnostic tests market. Hepatitis, characterized by liver inflammation, poses significant health risks and can lead to various symptoms and complications. Accurate diagnostic tests for hepatitis are crucial in identifying and confirming the presence of hepatitis infections, enabling timely interventions and effective disease management. For instance, according to the Hepatitis B Foundation in 2023, an estimated 1.5 million people contract hepatitis infections annually, highlighting the persistent challenge posed by these diseases. The growing incidence of hepatitis underlines the critical importance of reliable diagnostic tests in early detection and management, thereby fueling the demand for hepatitis diagnostic tests in the market.
Dominant players in the hepatitis diagnostic test market are concentrating on innovating automated immunoassays, exemplified by the Elecsys HCV Duo, to enhance customer service reliability. Automated immunoassays represent laboratory techniques utilizing automated systems and specialized instruments to conduct various phases of the immunoassay process, encompassing sample handling, reagent mixing, incubation, detection, and result calculation. For instance, in July 2023, Roche Diagnostics India, a biotechnology research company based in India, introduced Elecsys HCV Duo. This advanced solution represents India's maiden fully automated immunoassay available commercially, facilitating the simultaneous and independent evaluation of hepatitis C virus (HCV) antigen and antibody levels from a single human plasma or serum sample. Moreover, this approach streamlines the diagnostic procedure, enabling single-specimen testing and potentially reducing the number of necessary follow-up visits, consequently alleviating the burden on healthcare systems.
In October 2024, The Bank of Nova Scotia, a Canada-based company, acquired Bio-Rad Laboratories Inc. for an undisclosed amount. This acquisition allows The Bank of Nova Scotia to diversify its portfolio by integrating Bio-Rad Laboratories’ expertise in hepatitis diagnostic testing. Bio-Rad Laboratories Inc., a U.S.-based company, is known for its products and services in diagnostic testing for hepatitis.
Major companies operating in the hepatitis diagnostic tests market report are Thermo Fisher Scientific Inc., Abbott Laboratories, Danaher Corporation, Siemens Healthineers AG, Roche Diagnostics, Quest Diagnostics Incorporated, Grifols S.A, Hologic Inc., BioMérieux SA, Beckman Coulter Inc., Bio-Rad Laboratories Inc., SD Biosensor Co. Ltd., QIAGEN N.V., DiaSorin S.p.A., Randox Laboratories Ltd., Luminex Corporation, Fujirebio Diagnostics Inc., Trivitron Healthcare Private Limited, Trinity Biotech PLC, MP Biomedicals LLC, R-Biopharm AG, Creative Diagnostics, InBios International Inc., Dia.Pro Diagnostic Bioprobes srl, DIALAB GmbH.
North America was the largest region in the hepatitis diagnostic tests market in 2024. Asia-Pacific is expected to be the fastest-growing region in the forecast period. The regions covered in the hepatitis diagnostic tests market report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East, Africa. The countries covered in the hepatitis diagnostic tests market report are Australia, Brazil, China, France, Germany, India, Indonesia, Japan, Russia, South Korea, UK, USA, Canada, Italy, Spain.
The hepatitis diagnostic test market consists of revenues earned by entities by providing diagnostic services such as liver ultrasound, computerized tomography (CT) scans, and antibody tests. The market value includes the value of related goods sold by the service provider or included within the service offering. The hepatitis diagnostic test market also includes sales of laboratory equipment, reagents, and assay components that are used in providing hepatitis diagnostic test services. Values in this market are ‘factory gate’ values, that is, the value of goods sold by the manufacturers or creators of the goods, whether to other entities (including downstream manufacturers, wholesalers, distributors, and retailers) or directly to end customers. The value of goods in this market includes related services sold by the creators of the goods.
Hepatitis diagnostic tests are medical procedures and laboratory analyses designed to identify and diagnose infections caused by hepatitis viruses, including hepatitis A, B, C, D, and E. These tests are essential for detecting the presence of hepatitis viruses or specific antibodies in a patient's blood or other bodily fluids. The primary goal is to confirm the infection, determine its type, assess severity, and monitor its progression.
The main categories of hepatitis diagnostic tests include blood tests, imaging tests, and liver biopsy. Blood tests involve examining blood samples to assess various constituents such as blood cells, proteins, enzymes, hormones, antibodies, and other chemicals. Various technologies are utilized in these tests, including enzyme-linked immunosorbent assay (ELISA), rapid diagnostic tests, polymerase chain reaction (PCR), isothermal nucleic acid amplification tests, and others. These diagnostic tools find applications in diverse healthcare settings such as hospitals, diagnostic laboratories, blood banks, and other medical facilities.
The hepatitis diagnostic tests market research report is one of a series of new reports that provides hepatitis diagnostic tests market statistics, including hepatitis diagnostic tests industry global market size, regional shares, competitors with a hepatitis diagnostic tests market share, detailed hepatitis diagnostic tests market segments, market trends, and opportunities, and any further data you may need to thrive in the hepatitis diagnostic test industry. This hepatitis diagnostic tests market research report delivers a complete perspective of everything you need, with an in-depth analysis of the current and future scenario of the industry.
The market value is defined as the revenues that enterprises gain from the sale of goods and/or services within the specified market and geography through sales, grants, or donations in terms of the currency (in USD, unless otherwise specified).
The revenues for a specified geography are consumption values that are revenues generated by organizations in the specified geography within the market, irrespective of where they are produced. It does not include revenues from resales along the supply chain, either further along the supply chain or as part of other products.
This product will be delivered within 3-5 business days.
Table of Contents
Executive Summary
Hepatitis Diagnostic Tests Global Market Report 2025 provides strategists, marketers and senior management with the critical information they need to assess the market.This report focuses on hepatitis diagnostic tests market which is experiencing strong growth. The report gives a guide to the trends which will be shaping the market over the next ten years and beyond.
Reasons to Purchase:
- Gain a truly global perspective with the most comprehensive report available on this market covering 15 geographies.
- Assess the impact of key macro factors such as conflict, pandemic and recovery, inflation and interest rate environment and the 2nd Trump presidency.
- Create regional and country strategies on the basis of local data and analysis.
- Identify growth segments for investment.
- Outperform competitors using forecast data and the drivers and trends shaping the market.
- Understand customers based on the latest market shares.
- Benchmark performance against key competitors.
- Suitable for supporting your internal and external presentations with reliable high quality data and analysis
- Report will be updated with the latest data and delivered to you along with an Excel data sheet for easy data extraction and analysis.
- All data from the report will also be delivered in an excel dashboard format.
Description
Where is the largest and fastest growing market for hepatitis diagnostic tests? How does the market relate to the overall economy, demography and other similar markets? What forces will shape the market going forward? The hepatitis diagnostic tests market global report answers all these questions and many more.The report covers market characteristics, size and growth, segmentation, regional and country breakdowns, competitive landscape, market shares, trends and strategies for this market. It traces the market’s historic and forecast market growth by geography.
- The market characteristics section of the report defines and explains the market.
- The market size section gives the market size ($b) covering both the historic growth of the market, and forecasting its development.
- The forecasts are made after considering the major factors currently impacting the market. These include:
- The forecasts are made after considering the major factors currently impacting the market. These include the Russia-Ukraine war, rising inflation, higher interest rates, and the legacy of the COVID-19 pandemic.
- Market segmentations break down the market into sub markets.
- The regional and country breakdowns section gives an analysis of the market in each geography and the size of the market by geography and compares their historic and forecast growth. It covers the growth trajectory of COVID-19 for all regions, key developed countries and major emerging markets.
- The competitive landscape chapter gives a description of the competitive nature of the market, market shares, and a description of the leading companies. Key financial deals which have shaped the market in recent years are identified.
- The trends and strategies section analyses the shape of the market as it emerges from the crisis and suggests how companies can grow as the market recovers.
Scope
Markets Covered:
1) By Test Type: Blood Tests; Imaging Tests; Liver Biopsy2) By Technology: Enzyme-Linked Immunosorbent Assay (ELISA); Rapid Diagnostic Tests (RDTs); Polymerase Chain Reaction (PCR); Isothermal Nucleic Acid Amplification Test (INAAT); Other Technologies
3) By End User: Hospitals; Diagnostic Laboratories; Blood Bank; Other End Users
Subsegments:
1) By Blood Tests: Serological Tests; Polymerase Chain Reaction (PCR) Tests; Enzyme-Linked Immunosorbent Assays (ELISA)2) By Imaging Tests: Ultrasound; Computed Tomography (CT); Magnetic Resonance Imaging (MRI)
3) By Liver Biopsy: Percutaneous Biopsy; Transjugular Biopsy; Laparoscopic Biopsy
Key Companies Mentioned: Thermo Fisher Scientific Inc.; Abbott Laboratories; Danaher Corporation; Siemens Healthineers AG; Roche Diagnostics
Countries: Australia; Brazil; China; France; Germany; India; Indonesia; Japan; Russia; South Korea; UK; USA; Canada; Italy; Spain
Regions: Asia-Pacific; Western Europe; Eastern Europe; North America; South America; Middle East; Africa
Time Series: Five years historic and ten years forecast.
Data: Ratios of market size and growth to related markets, GDP proportions, expenditure per capita.
Data Segmentation: Country and regional historic and forecast data, market share of competitors, market segments.
Sourcing and Referencing: Data and analysis throughout the report is sourced using end notes.
Delivery Format: PDF, Word and Excel Data Dashboard.
Companies Mentioned
Some of the major companies featured in this Hepatitis Diagnostic Tests market report include:- Thermo Fisher Scientific Inc.
- Abbott Laboratories
- Danaher Corporation
- Siemens Healthineers AG
- Roche Diagnostics
- Quest Diagnostics Incorporated
- Grifols S.A
- Hologic Inc.
- BioMérieux SA
- Beckman Coulter Inc.
- Bio-Rad Laboratories Inc.
- SD Biosensor Co. Ltd.
- QIAGEN N.V.
- DiaSorin S.p.A.
- Randox Laboratories Ltd.
- Luminex Corporation
- Fujirebio Diagnostics Inc.
- Trivitron Healthcare Private Limited
- Trinity Biotech plc
- MP Biomedicals LLC
- R-Biopharm AG
- Creative Diagnostics
- InBios International Inc.
- Dia.Pro Diagnostic Bioprobes srl
- DIALAB GmbH
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 200 |
| Published | March 2025 |
| Forecast Period | 2025 - 2029 |
| Estimated Market Value ( USD | $ 4.95 Billion |
| Forecasted Market Value ( USD | $ 6.24 Billion |
| Compound Annual Growth Rate | 6.0% |
| Regions Covered | Global |
| No. of Companies Mentioned | 26 |