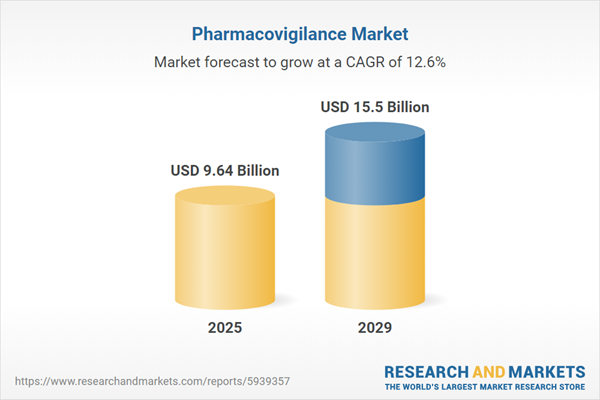
The pharmacovigilance market size is expected to see rapid growth in the next few years. It will grow to $15.5 billion in 2029 at a compound annual growth rate (CAGR) of 12.6%. The growth in the forecast period can be attributed to the rising healthcare expenditure, the increasing government support and the aging population. Major trends in the forecast period include increasing investments, use of artificial intelligence, use of innovative platforms, use of innovative cloud-based systems, and strategic mergers and acquisitions.
The forecast of 12.6% growth over the next five years reflects a modest reduction of 0.3% from the previous estimate for this market. This reduction is primarily due to the impact of tariffs between the US and other countries. Tariff barriers are expected to hamper drug safety monitoring by increasing the cost of adverse event reporting systems and signal detection software from UK-based providers, thereby delaying risk assessments and raising post-market surveillance costs. The effect will also be felt more widely due to reciprocal tariffs and the negative effect on the global economy and trade due to increased trade tensions and restrictions.
The rise in research and development activities is expected to propel the pharmacovigilance market. By investing in R&D, pharmaceutical companies can create new and innovative drugs with enhanced safety profiles. The rigorous preclinical and clinical testing during the drug development process allows for the early identification of potential safety issues, enabling the implementation of effective risk mitigation strategies. Furthermore, R&D efforts advance technologies for monitoring adverse events, data analysis, and signal detection, which facilitate the early identification of adverse drug reactions during the post-marketing phase. For example, in February 2024, the Llywodraeth Cymru Welsh Government, a UK-based agency, reported that Wales invested £1 billion ($1.25 billion) in Business Expenditure on Research and Development (BERD) in 2022, representing 1.9% of the total UK expenditure in this area. Thus, the growth in research and development activities is driving the pharmacovigilance market.
The increase in healthcare expenditure is expected to drive the growth of the pharmacovigilance market during the forecast period. With additional funding, healthcare systems can invest in advanced technologies, data analytics, and dedicated expert teams to effectively identify and assess adverse drug reactions. Moreover, greater healthcare spending facilitates better training and education for healthcare professionals on pharmacovigilance practices, ensuring timely reporting and analysis of potential drug-related issues. For example, in June 2024, The King's Fund, a UK-based non-profit organization, reported that the UK allocated 11.3% of its GDP to healthcare in 2022, slightly above the average for similar countries. This represents an increase from the pre-COVID-19 pandemic level of 9.9% of GDP dedicated to health services. This rise in expenditure underscores a growing emphasis on healthcare services in response to the pandemic, indicating a significant shift in funding priorities. Therefore, the increasing healthcare expenditure will support the growth of the pharmacovigilance market in the future.
Companies in the pharmacovigilance market are increasingly leveraging artificial intelligence (AI) to enhance efficiency. AI technology can significantly improve the accuracy and efficiency of pharmacovigilance activities, ultimately resulting in better patient outcomes. By incorporating AI, pharmaceutical companies can streamline processes, enhance data management, and expedite drug discovery, all while reducing costs and increasing safety. For example, in August 2024, the Government of India introduced the Adverse Drug Reaction Monitoring System (ADRMS) software, unveiled by the Minister of Health & Family Welfare and the Minister of Chemicals and Fertilizers. This pharmacovigilance software aids in the collection and analysis of adverse events related to medicines and medical devices, simplifying the reporting process for both consumers and healthcare professionals. This initiative considerably bolsters India's pharmacovigilance infrastructure by ensuring a more thorough capture of safety data and enhancing drug safety monitoring. The ADRMS aims to improve the efficiency of drug safety oversight and compliance with regulatory standards, positioning India as a leader in the global pharmaceutical industry.
Pharmacovigilance providers are making substantial investments to expand their product portfolios and geographic presence. In September 2022, the PharmaLex Group opened a new branch in Beijing, China, offering comprehensive pharmacovigilance, regulatory affairs, development advice, and quality management services to global companies entering the Chinese market. The PharmaLex Group's expansion aims to provide enhanced support and value across the product lifecycle.
In January 2023, AmerisourceBergen acquired Pharma Lex to augment its existing service offering and provide pharmaceutical and biotech companies with comprehensive support for pharmacovigilance systems and operations, including benefit-risk management services. These strategic investments contribute to the growth and expansion of the pharmacovigilance market.
Major companies operating in the pharmacovigilance market include IQVIA, Cognizant, ICON Plc, Accenture plc, PAREXEL International Corporation., United BioSource LLC, ArisGlobal, Quanticate, Wipro Limited, Linical Americas, Novotech CRO, Wuxi Apptec, Simcere Pharmaceutical Group, Lee's Pharmaceutical Holdings, Luye Pharma Group, EXTEDO, Arriello, PrimeVigilance, Axios International, C3i Solutions, Alcon, Secure AI Labs, OmniSol dot Tech, Canna Call Company, Veripad, Prevnos Inc., Lifescient, Inc, BeiGene, QuintilesIMS, Labcorp Drug Development, Pharmaceutical Product Development Inc., PRA Health Sciences, Synowledge LLC, RAPAhub, illuminate health, Invenio Medical, ClinChoice Inc., MSD, Novasyte, Fortrea Holdings Inc, Syneos Health Clinical, Zoetis Inc., Telerx Marketing Inc., Gilead Sciences, Inc., Elanco, Abbott Laboratories, Spimaco, Teva Pharmaceutical Industries Ltd.
North America was the largest region in the pharmacovigilance market in 2024. Asia-Pacific is expected to be the fastest-growing region in the forecast region. The regions covered in the pharmacovigilance market report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East, Africa. The countries covered in the pharmacovigilance market report are Australia, Brazil, China, France, Germany, India, Indonesia, Japan, Russia, South Korea, UK, USA, Italy, Spain, Canada.
Note that the outlook for this market is being affected by rapid changes in trade relations and tariffs globally. The report will be updated prior to delivery to reflect the latest status, including revised forecasts and quantified impact analysis. The report’s Recommendations and Conclusions sections will be updated to give strategies for entities dealing with the fast-moving international environment.
The sudden escalation of U.S. tariffs and the resulting trade tensions in spring 2025 are having a significant impact on the pharmaceutical sector. Companies are grappling with higher costs on imported active pharmaceutical ingredients (APIs), glass vials, and laboratory equipment - many of which have limited alternative sources. Generic drug manufacturers, already operating with minimal profit margins, are particularly affected, with some scaling back production of low-margin medications. Biotech firms are also experiencing delays in clinical trials due to shortages of specialized reagents linked to tariffs. In response, the industry is shifting API production to regions like India and Europe, building up inventory reserves, and advocating for tariff exemptions on essential medicines.
The pharmacovigilance market research report is one of a series of new reports that provides pharmacovigilance market statistics, including pharmacovigilance industry global market size, regional shares, competitors with a pharmacovigilance market share, detailed pharmacovigilance market segments, market trends, and opportunities, and any further data you may need to thrive in the pharmacovigilance industry. This pharmacovigilance market research report delivers a complete perspective of everything you need, with an in-depth analysis of the current and future scenarios of the industry.
Pharmacovigilance encompasses the science and practices dedicated to identifying, evaluating, understanding, and mitigating side effects and other medical/vaccination-related issues. Its purpose is to assess whether the benefits of a drug outweigh its risks, and this scrutiny persists even after drug approval.
The primary types of pharmacovigilance include spontaneous reporting, intensified adverse drug reaction (ADR) reporting, targeted spontaneous reporting, cohort event monitoring, and electronic health record (EHR) mining. Spontaneous reporting relies on individuals' voluntary reporting of suspected adverse drug reactions to local or national pharmacovigilance centers, representing a passive approach. Process flows involve case data management, signal detection, and risk management systems. Service providers can be in-house or contracted for outsourcing, covering various clinical trial phases such as preclinical, phase I, phase II, phase III, and phase IV. These services cater to different end-users, including hospitals, pharmaceutical companies, and others.
The pharmacovigilance market includes revenues earned by collecting, analyzing, monitoring, and preventing adverse effects in drugs and therapies. The market value includes the value of related goods sold by the service provider or included within the service offering. Only goods and services traded between entities or sold to end consumers are included.
The market value is defined as the revenues that enterprises gain from the sale of goods and/or services within the specified market and geography through sales, grants, or donations in terms of the currency (in USD unless otherwise specified).
The revenues for a specified geography are consumption values that are revenues generated by organizations in the specified geography within the market, irrespective of where they are produced. It does not include revenues from resales along the supply chain, either further along the supply chain or as part of other products.
This product will be delivered within 1-3 business days.
Table of Contents
Executive Summary
Pharmacovigilance Global Market Report 2025 provides strategists, marketers and senior management with the critical information they need to assess the market.This report focuses on pharmacovigilance market which is experiencing strong growth. The report gives a guide to the trends which will be shaping the market over the next ten years and beyond.
Reasons to Purchase:
- Gain a truly global perspective with the most comprehensive report available on this market covering 15 geographies.
- Assess the impact of key macro factors such as geopolitical conflicts, trade policies and tariffs, post-pandemic supply chain realignment, inflation and interest rate fluctuations, and evolving regulatory landscapes.
- Create regional and country strategies on the basis of local data and analysis.
- Identify growth segments for investment.
- Outperform competitors using forecast data and the drivers and trends shaping the market.
- Understand customers based on the latest market shares.
- Benchmark performance against key competitors.
- Suitable for supporting your internal and external presentations with reliable high quality data and analysis
- Report will be updated with the latest data and delivered to you along with an Excel data sheet for easy data extraction and analysis.
- All data from the report will also be delivered in an excel dashboard format.
Description
Where is the largest and fastest growing market for pharmacovigilance? How does the market relate to the overall economy, demography and other similar markets? What forces will shape the market going forward, including technological disruption, regulatory shifts, and changing consumer preferences? The pharmacovigilance market global report answers all these questions and many more.The report covers market characteristics, size and growth, segmentation, regional and country breakdowns, competitive landscape, market shares, trends and strategies for this market. It traces the market’s historic and forecast market growth by geography.
- The market characteristics section of the report defines and explains the market.
- The market size section gives the market size ($b) covering both the historic growth of the market, and forecasting its development.
- The forecasts are made after considering the major factors currently impacting the market. These include: the technological advancements such as AI and automation, Russia-Ukraine war, trade tariffs (government-imposed import/export duties), elevated inflation and interest rates.
- Market segmentations break down the market into sub markets.
- The regional and country breakdowns section gives an analysis of the market in each geography and the size of the market by geography and compares their historic and forecast growth.
- The competitive landscape chapter gives a description of the competitive nature of the market, market shares, and a description of the leading companies. Key financial deals which have shaped the market in recent years are identified.
- The trends and strategies section analyses the shape of the market as it emerges from the crisis and suggests how companies can grow as the market recovers.
Scope
Markets Covered:
1) By Type: Spontaneous Reporting, Intensified ADR Reporting, Targeted Spontaneous Reporting, Cohort Event Monitoring, EHR Mining2) By Service Provider: in-House, Contract Outsourcing
3) By Process Flow: Case Data Management, Signal Detection, Risk Management System
4) By Clinical Trial Phases: Preclinical, Phase I, Phase II, Phase III, Phase IV
5) By End User: Hospitals, Pharmaceutical Companies, Other End Users
Subsegments:
1) By Spontaneous Reporting: Individual Case Safety Reports (ICSRs); Voluntary Reporting Systems; Case Reports From Healthcare Professionals2) By Intensified ADR Reporting: Active Surveillance Programs; Enhanced Monitoring in Clinical Trials; Risk Minimization Strategies
3) By Targeted Spontaneous Reporting: Reporting for Specific Drug Classes; Condition-Specific Reporting; Focused Surveillance Programs
4) By Cohort Event Monitoring: Prospective Cohort Studies; Retrospective Cohort Studies; Longitudinal Studies
5) By EHR Mining: Data Extraction From Electronic Health Records; Signal Detection Through EHR Analysis; Integration of EHR Data With Pharmacovigilance Systems
Companies Mentioned: IQVIA; Cognizant; ICON Plc; Accenture plc; PAREXEL International Corporation.; United BioSource LLC; ArisGlobal; Quanticate; Wipro Limited; Linical Americas; Novotech CRO; Wuxi Apptec; Simcere Pharmaceutical Group; Lee's Pharmaceutical Holdings; Luye Pharma Group; EXTEDO; Arriello; PrimeVigilance; Axios International; C3i Solutions; Alcon; Secure AI Labs; OmniSol dot Tech; Canna Call Company; Veripad; Prevnos Inc.; Lifescient, Inc; BeiGene; QuintilesIMS; Labcorp Drug Development; Pharmaceutical Product Development Inc.; PRA Health Sciences; Synowledge LLC; RAPAhub; illuminate health; Invenio Medical; ClinChoice Inc.; MSD; Novasyte; Fortrea Holdings Inc; Syneos Health Clinical; Zoetis Inc.; Telerx Marketing Inc.; Gilead Sciences, Inc.; Elanco; Abbott Laboratories; Spimaco; Teva Pharmaceutical Industries Ltd.
Countries: Australia; Brazil; China; France; Germany; India; Indonesia; Japan; Russia; South Korea; UK; USA; Canada; Italy; Spain
Regions: Asia-Pacific; Western Europe; Eastern Europe; North America; South America; Middle East; Africa
Time Series: Five years historic and ten years forecast.
Data: Ratios of market size and growth to related markets, GDP proportions, expenditure per capita.
Data Segmentation: Country and regional historic and forecast data, market share of competitors, market segments.
Sourcing and Referencing: Data and analysis throughout the report is sourced using end notes.
Delivery Format: PDF, Word and Excel Data Dashboard.
Companies Mentioned
The companies featured in this Pharmacovigilance market report include:- IQVIA
- Cognizant
- ICON Plc
- Accenture plc
- PAREXEL International Corporation.
- United BioSource LLC
- ArisGlobal
- Quanticate
- Wipro Limited
- Linical Americas
- Novotech CRO
- Wuxi Apptec
- Simcere Pharmaceutical Group
- Lee's Pharmaceutical Holdings
- Luye Pharma Group
- EXTEDO
- Arriello
- PrimeVigilance
- Axios International
- C3i Solutions
- Alcon
- Secure AI Labs
- OmniSol dot Tech
- Canna Call Company
- Veripad
- Prevnos Inc.
- Lifescient, Inc
- BeiGene
- QuintilesIMS
- Labcorp Drug Development
- Pharmaceutical Product Development Inc.
- PRA Health Sciences
- Synowledge LLC
- RAPAhub
- illuminate health
- Invenio Medical
- ClinChoice Inc.
- MSD
- Novasyte
- Fortrea Holdings Inc
- Syneos Health Clinical
- Zoetis Inc.
- Telerx Marketing Inc.
- Gilead Sciences, Inc.
- Elanco
- Abbott Laboratories
- Spimaco
- Teva Pharmaceutical Industries Ltd.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 250 |
| Published | September 2025 |
| Forecast Period | 2025 - 2029 |
| Estimated Market Value ( USD | $ 9.64 Billion |
| Forecasted Market Value ( USD | $ 15.5 Billion |
| Compound Annual Growth Rate | 12.6% |
| Regions Covered | Global |
| No. of Companies Mentioned | 49 |