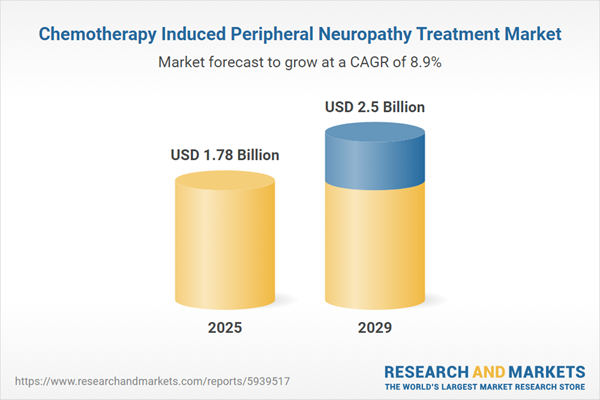
The chemotherapy induced peripheral neuropathy treatment market size is expected to see strong growth in the next few years. It will grow to $2.5 billion in 2029 at a compound annual growth rate (CAGR) of 8.9%. The growth in the forecast period can be attributed to targeted therapies development, neuroprotective agents, integrated care pathways, patient-centered care models, regulatory support. Major trends in the forecast period include novel drug formulations, non-pharmacological interventions, neuroplasticity-based therapies, long-term survivorship support, cognitive behavioral therapies.
The growth trajectory of the chemotherapy-induced peripheral neuropathy treatment market is anticipated to be driven by the increasing prevalence of cancer. Cancer, characterized by the unregulated proliferation and dissemination of abnormal cells within the body, often necessitates chemotherapy treatment. However, one of the potential side effects of certain chemotherapy drugs is chemotherapy-induced peripheral neuropathy (CIPN). The rising incidence of cancer cases globally is expected to contribute significantly to the expansion of the chemotherapy-induced peripheral neuropathy treatment market. For instance, a report by the American Cancer Society in January 2023 revealed an increase in newly diagnosed cases of liver cancer in females in the United States, rising from 12,660 cases in 2022 to 13,230 cases in 2023. This escalation in cancer cases acts as a catalyst propelling the growth of the chemotherapy-induced peripheral neuropathy treatment market.
The expansion of the chemotherapy-induced peripheral neuropathy treatment market is being driven by an increase in healthcare spending. Healthcare spending, which refers to the total financial resources allocated to the healthcare sector in a specific region or country, is essential for supporting treatments for chemotherapy-induced peripheral neuropathy (CIPN). This funding aims to alleviate and manage the adverse neurological effects caused by certain cancer therapies, ensuring the well-being of patients undergoing and recovering from chemotherapy. For example, in May 2023, a report from the Office for National Statistics, a UK government department, indicated that healthcare spending in the UK rose by 5.6% from 2022 to 2023, compared to a growth of 0.9% in 2022. The UK’s healthcare expenditure was approximately $317.63 billion (£292 billion) in 2023. This increase in healthcare expenditure acts as a catalyst for the growth of the chemotherapy-induced peripheral neuropathy treatment market in the future.
Product innovation emerges as a prominent trend in the chemotherapy-induced peripheral neuropathy treatment market, with major market players prioritizing the development of novel products to enhance efficacy and consolidate their market position. Notably, in March 2023, AlgoTx, a clinical-stage company specializing in complex pain management, announced the FDA's approval of their Investigational New Drug Application (IND) for ATX01, a Phase 2 first-in-class candidate designed for individuals with erythromelalgia. The ATX01 for the Pain of Chemotherapy (ACT) study targeting chemotherapy-induced peripheral neuropathy (CIPN) has commenced randomized patient enrollment. Both programs have secured fast-track designation from the FDA, signifying their potential significance in addressing unmet medical needs.
Technological advancements, particularly the incorporation of quell technology, are a focal point for major companies in the chemotherapy-induced peripheral neuropathy treatment market as they endeavor to fortify their market standing. For instance, in January 2022, NeuroMetrix Inc., a US-based provider of non-invasive medical devices, attained breakthrough device designation from the U.S. Food and Drug Administration (FDA) for the mitigation of moderate to severe symptoms of chemotherapy-induced peripheral neuropathy (CIPN) persisting for at least 6 months post-chemotherapy completion. This designation marks a pivotal milestone for NeuroMetrix's efforts to introduce Quell technology to alleviate the distressing effects experienced by individuals grappling with CIPN.
In August 2024, Pharmacosmos Group, an international healthcare company based in Denmark, acquired G1 Therapeutics for $405 million. The purpose of this acquisition is to expand and accelerate the availability of COSELA® (trilaciclib), a treatment aimed at reducing chemotherapy-induced myelosuppression in patients with extensive-stage small cell lung cancer (ES-SCLC). G1 Therapeutics is a US-based biopharmaceutical company that specializes in developing and commercializing small molecule therapeutics for cancer treatment.
Major companies operating in the chemotherapy induced peripheral neuropathy treatment market include Hoffmann-La Roche Ltd., Midatech Pharma PLC, Novartis AG, Lee's Pharmaceutical Holdings Limited, RELIEF THERAPEUTICS Holding SA, Eurofins Advinus, WEX Pharmaceuticals Inc., Asahi Kasei Corporation, MediciNova Inc., Solasia Pharma K.K., ESTEVE, ChromaDex Inc., Apollo Endosurgery Inc., REGENACY PHARMACEUTICALS INC., Novaremed AG, MAKScientific LLC, Sova Pharmaceuticals Inc., Kineta Inc., Aptinyx Inc., Apexian Pharmaceuticals Inc., WinSanTor Inc., Alexion Pharmaceuticals Inc., Sanofi, Takeda Pharmaceutical Company Limited, Vertex Pharmaceuticals Incorporated, Abbott Laboratories, Acorda Therapeutics Inc., Galena Biopharma Inc., Incyte Corporation.
North America was the largest region in the chemotherapy induced peripheral neuropathy treatment market in 2024. Asia-Pacific and Europe are expected to be the fastest-growing regions in the forecast period. The regions covered in the chemotherapy induced peripheral neuropathy treatment market report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East, Africa. The countries covered in the chemotherapy induced peripheral neuropathy treatment market report are Australia, Brazil, China, France, Germany, India, Indonesia, Japan, Russia, South Korea, UK, USA, Canada, Italy, Spain.
Chemotherapy Induced Peripheral Neuropathy (CIPN) encompasses a range of symptoms and complications arising from specific chemotherapy drugs utilized in cancer treatment. It represents a form of peripheral neuropathy, involving impairment or dysfunction of the nerves connecting the brain and spinal cord to the body.
The primary drug categories for CIPN treatment include nerve protective therapy, anti-inflammatory therapy, neurotransmitter-based therapy, antioxidants, and others. Nerve protective therapy aims to preserve nerve health and function, crucial in managing chemotherapy-induced peripheral neuropathy for symptom relief. Treatment modalities encompass medications, therapies, and other interventions, with both branded and generic drugs available. These CIPN treatment drugs are distributed through hospital pharmacies, retail outlets, online platforms, and other channels, catering to end-users such as hospitals, research institutes, and specialty clinics.
The chemotherapy induced peripheral neuropathy treatment market research report is one of a series of new reports that provides chemotherapy induced peripheral neuropathy treatment market statistics, including the chemotherapy induced peripheral neuropathy treatment industry's global market size, regional shares, competitors with an chemotherapy induced peripheral neuropathy treatment market share, detailed chemotherapy induced peripheral neuropathy treatment market segments, market trends, and opportunities, and any further data you may need to thrive in the chemotherapy induced peripheral neuropathy treatment industry. This chemotherapy induced peripheral neuropathy treatment market research report delivers a complete perspective of everything you need, with an in-depth analysis of the current and future scenarios of the industry.
The chemotherapy induced peripheral neuropathy treatment market consists of sales of pharmaceutical drugs prescribed to alleviate chemotherapy-induced peripheral neuropathy symptoms. These drugs include pain relievers, antidepressants, anticonvulsants, and topical creams. Values in this market are ‘factory gate’ values, that is the value of goods sold by the manufacturers or creators of the goods, whether to other entities (including downstream manufacturers, wholesalers, distributors, and retailers) or directly to end customers. The value of goods in this market includes related services sold by the creators of the goods.
The market value is defined as the revenues that enterprises gain from the sale of goods and/or services within the specified market and geography through sales, grants, or donations in terms of the currency (in USD, unless otherwise specified).
The revenues for a specified geography are consumption values that are revenues generated by organizations in the specified geography within the market, irrespective of where they are produced. It does not include revenues from resales along the supply chain, either further along the supply chain or as part of other products.
This product will be delivered within 3-5 business days.
Table of Contents
Executive Summary
Chemotherapy Induced Peripheral Neuropathy Treatment Global Market Report 2025 provides strategists, marketers and senior management with the critical information they need to assess the market.This report focuses on chemotherapy induced peripheral neuropathy treatment market which is experiencing strong growth. The report gives a guide to the trends which will be shaping the market over the next ten years and beyond.
Reasons to Purchase:
- Gain a truly global perspective with the most comprehensive report available on this market covering 15 geographies.
- Assess the impact of key macro factors such as conflict, pandemic and recovery, inflation and interest rate environment and the 2nd Trump presidency.
- Create regional and country strategies on the basis of local data and analysis.
- Identify growth segments for investment.
- Outperform competitors using forecast data and the drivers and trends shaping the market.
- Understand customers based on the latest market shares.
- Benchmark performance against key competitors.
- Suitable for supporting your internal and external presentations with reliable high quality data and analysis
- Report will be updated with the latest data and delivered to you along with an Excel data sheet for easy data extraction and analysis.
- All data from the report will also be delivered in an excel dashboard format.
Description
Where is the largest and fastest growing market for chemotherapy induced peripheral neuropathy treatment? How does the market relate to the overall economy, demography and other similar markets? What forces will shape the market going forward? The chemotherapy induced peripheral neuropathy treatment market global report answers all these questions and many more.The report covers market characteristics, size and growth, segmentation, regional and country breakdowns, competitive landscape, market shares, trends and strategies for this market. It traces the market’s historic and forecast market growth by geography.
- The market characteristics section of the report defines and explains the market.
- The market size section gives the market size ($b) covering both the historic growth of the market, and forecasting its development.
- The forecasts are made after considering the major factors currently impacting the market. These include:
- The forecasts are made after considering the major factors currently impacting the market. These include the Russia-Ukraine war, rising inflation, higher interest rates, and the legacy of the COVID-19 pandemic.
- Market segmentations break down the market into sub markets.
- The regional and country breakdowns section gives an analysis of the market in each geography and the size of the market by geography and compares their historic and forecast growth. It covers the growth trajectory of COVID-19 for all regions, key developed countries and major emerging markets.
- The competitive landscape chapter gives a description of the competitive nature of the market, market shares, and a description of the leading companies. Key financial deals which have shaped the market in recent years are identified.
- The trends and strategies section analyses the shape of the market as it emerges from the crisis and suggests how companies can grow as the market recovers.
Scope
Markets Covered:
1) By Drug Class: Nerve Protective Therapy; Anti-Inflammatory Therapy; Neurotransmitter Based Therapy; Antioxidant; Other Drug Classes2) By Treatment: Medication; Therapy; Other Treatments
3) By Drug Type: Branded; Generic
4) By Distribution Channel: Hospital Pharmacy; Retail Pharmacy; Online Pharmacies; Other Distribution Channels
5) By End-User: Hospitals; Research Institutes; Specialty Clinics
Subsegments:
1) By Nerve Protective Therapy: Vitamin B Complex; Alpha-Lipoic Acid; Acetyl-L-Carnitine2) By Anti-Inflammatory Therapy: Corticosteroids; Non-Steroidal Anti-Inflammatory Drugs (NSAIDs)
3) By Neurotransmitter Based Therapy: Gabapentin; Pregabalin; Duloxetine
4) By Antioxidant: N-acetylcysteine; Glutathione; Coenzyme Q10
5) By Other Drug Classes: Topical Treatments; Opioids; Herbal Remedies
Key Companies Mentioned: Hoffmann-La Roche Ltd.; Midatech Pharma PLC; Novartis AG; Lee's Pharmaceutical Holdings Limited; RELIEF THERAPEUTICS Holding SA
Countries: Australia; Brazil; China; France; Germany; India; Indonesia; Japan; Russia; South Korea; UK; USA; Canada; Italy; Spain
Regions: Asia-Pacific; Western Europe; Eastern Europe; North America; South America; Middle East; Africa
Time Series: Five years historic and ten years forecast.
Data: Ratios of market size and growth to related markets, GDP proportions, expenditure per capita.
Data Segmentation: Country and regional historic and forecast data, market share of competitors, market segments.
Sourcing and Referencing: Data and analysis throughout the report is sourced using end notes.
Delivery Format: PDF, Word and Excel Data Dashboard.
Companies Mentioned
- Hoffmann-La Roche Ltd.
- Midatech Pharma PLC
- Novartis AG
- Lee's Pharmaceutical Holdings Limited
- RELIEF THERAPEUTICS Holding SA
- Eurofins Advinus
- WEX Pharmaceuticals Inc.
- Asahi Kasei Corporation
- MediciNova Inc.
- Solasia Pharma K.K.
- ESTEVE
- ChromaDex Inc.
- Apollo Endosurgery Inc.
- REGENACY PHARMACEUTICALS INC.
- Novaremed AG
- MAKScientific LLC
- Sova Pharmaceuticals Inc.
- Kineta Inc.
- Aptinyx Inc.
- Apexian Pharmaceuticals Inc.
- WinSanTor Inc.
- Alexion Pharmaceuticals Inc.
- Sanofi
- Takeda Pharmaceutical Company Limited
- Vertex Pharmaceuticals Incorporated
- Abbott Laboratories
- Acorda Therapeutics Inc.
- Galena Biopharma Inc.
- Incyte Corporation
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 200 |
| Published | February 2025 |
| Forecast Period | 2025 - 2029 |
| Estimated Market Value ( USD | $ 1.78 Billion |
| Forecasted Market Value ( USD | $ 2.5 Billion |
| Compound Annual Growth Rate | 8.9% |
| Regions Covered | Global |
| No. of Companies Mentioned | 29 |