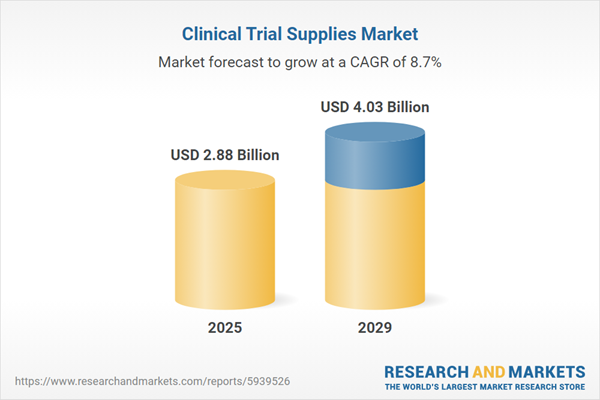
The clinical trial supplies market size is expected to see strong growth in the next few years. It will grow to $4.03 billion in 2029 at a compound annual growth rate (CAGR) of 8.7%. The growth in the forecast period can be attributed to globalization of clinical trials, expansion of cell and gene therapy trials, increased focus on patient-centric trials, growing number of biologics and biosimilar drugs in trials, rise of virtual and decentralized trials. Major trends in the forecast period include integration of artificial intelligence (AI) and predictive analytics, 3D printing, increasing focus on sustainability and eco-friendliness in clinical trial supply management, growing R&D investments by biopharmaceutical and pharmaceutical companies, blockchain technology advancements.
The clinical trial supplies market is poised for substantial growth, driven by the escalating number of registered clinical trials. These trials, essential for assessing the safety and efficacy of new medical treatments, interventions, and diagnostic procedures, rely on a range of supplies, including pharmaceuticals and medical devices. To illustrate, in May 2023, ClinicalTrials.gov reported a notable increase, with 437,533 registered clinical trials compared to 399,499 in 2022 across the United States and 221 countries. This surge in clinical trial registrations is a key factor fueling the expansion of the clinical trial supplies market, crucial for ensuring successful trials, accurate data collection, and patient safety.
The clinical trial supplies market is expected to experience significant growth, propelled by the expanding geriatric population. The term 'geriatric population' refers to individuals aged 65 and older, often dealing with multiple health conditions. This demographic shift creates a heightened demand for diverse and specialized trial supplies to support research and development efforts focused on age-related diseases. As of October 2022, a report from the World Health Organization projects that by 2030, one out of every six individuals globally will be 60 years old or older. By 2050, the number is expected to reach 2.1 billion. Consequently, the growing geriatric population plays a pivotal role in driving the clinical trial supplies market's growth.
A prominent trend shaping the landscape of the clinical trial supplies market is the adoption of advanced real-world evidence platforms. Leading companies in this market are embracing innovative technologies to revolutionize healthcare decision-making, drug development, and patient care. This strategic move involves harnessing real-world data to generate evidence-based insights. As an illustration, in April 2023, Komodo Health, a US-based healthcare technology company, introduced MapEnhance - an integrated real-world evidence technology platform. This platform, incorporating a network of specialty data partners, provides customers with a comprehensive perspective on clinical events and patient populations. MapEnhance includes precision molecular diagnostics, standard laboratory diagnostics, and electronic medical records, offering unparalleled insights for various therapeutic areas and hospital settings.
Major players in the clinical trial supplies market are actively investing in cutting-edge technologies, such as clinical development platforms, to broaden their customer base, drive sales, and enhance revenue. A clinical development platform serves as an integrated system or software facilitating the planning, management, execution, and monitoring of clinical trials and research studies. For instance, in June 2022, ZS Associates, a US-based management consulting and technology firm, launched the ZAIDYN Clinical Development platform. This platform stands out for its ability to break down silos in clinical trial design, overcome enrollment and engagement obstacles, and expedite product delivery to patients. ZAIDYN facilitates seamless global team collaboration, connecting products, processes, applications, algorithms, and data assets to provide potent insights influencing clinical research. Its compatibility with existing IT systems enhances efficiency and connectivity, reducing costs and empowering life sciences companies of all sizes to innovate and thrive in the evolving global healthcare ecosystem.
In May 2024, Myonex, a pharmaceutical company based in the US, acquired Creapharm’s pharmaceutical services for an undisclosed amount. Following this acquisition, Creapharm's capabilities in clinical packaging, distribution, commercial packaging, and bioservices will operate under the name Creapharm, a Myonex company. This merger enhances flexibility and strengthens service offerings for pharmaceutical and biotech companies at both clinical and commercial stages, including those involved with Advanced Therapies. Creapharm is a manufacturer of clinical trial supplies based in France.
Major companies operating in the clinical trial supplies market include Thermo Fisher Scientific Inc., IQVIA, Eurofins Scientific SE, Parexel International Corporation, ICON PLC, Catalent Inc., Intertek Group PLC, Recipharm AB, World Courier, Almac Group Ltd., Piramal Pharma Solutions, Clinigen Group PLC, Movianto GmbH, Marken Limited, PCI Pharma Services, Rubicon Research Private Limited, Bionical Ltd., Durbin PLC, SIRO Clinpharm Pvt. Ltd., Biocair International Ltd., Ancillare LP., Myonex, Klifo A/S, Alium Medical Limited, ADAllen Pharma, Sharp Services LLC.
North America was the largest region in the clinical trial supplies market in 2024. Asia-Pacific is expected to be the fastest-growing region in the forecast period. The regions covered in the clinical trial supplies market report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East, Africa. The countries covered in the clinical trial supplies market report are Australia, Brazil, China, France, Germany, India, Indonesia, Japan, Russia, South Korea, UK, USA, Canada, Italy, Spain.
Clinical trial supplies encompass materials, products, and equipment pivotal for the smooth conduct of clinical trials. They are instrumental in facilitating testing and evaluation processes.
Essential services related to clinical trial supplies involve logistics and distribution, storage and retention, supply chain management, packaging, labeling, blinding manufacturing, and comparator sourcing. Logistics and distribution manage the movement of trial supplies from manufacturing facilities to trial sites, ensuring prompt availability and efficient delivery. These supplies are crucial across various clinical phases (Phase I to Phase IV) for treating conditions such as oncology, CNS disorders, cardiovascular ailments, infectious diseases, metabolic disorders, among others. End users include pharmaceutical and biotech companies, contract research organizations (CROs), and medical device firms.
The clinical trial supplies research report is one of a series of new reports that provides clinical trial supplies market statistics, including the clinical trial supplies industry's global market size, regional shares, competitors with a clinical trial supplies market share, detailed clinical trial supplies market segments, market trends and opportunities, and any further data you may need to thrive in the clinical trial supplies industry. This clinical trial supplies market research report delivers a complete perspective of everything you need, with an in-depth analysis of the current and future scenarios of the industry.
The clinical trial supplies market consists of revenues earned by entities by providing quality control, regulatory compliance, and return and destruction management services. The market value includes the value of related goods sold by the service provider or included within the service offering. The clinical trial supplies market also includes sales of investigational products, comparator products, and clinical trial kits. Values in this market are ‘factory gate’ values, that is the value of goods sold by the manufacturers or creators of the goods, whether to other entities (including downstream manufacturers, wholesalers, distributors, and retailers) or directly to end customers. The value of goods in this market includes related services sold by the creators of the goods.
The market value is defined as the revenues that enterprises gain from the sale of goods and/or services within the specified market and geography through sales, grants, or donations in terms of the currency (in USD, unless otherwise specified).
The revenues for a specified geography are consumption values that are revenues generated by organizations in the specified geography within the market, irrespective of where they are produced. It does not include revenues from resales along the supply chain, either further along the supply chain or as part of other products.
This product will be delivered within 3-5 business days.
Table of Contents
Executive Summary
Clinical Trial Supplies Global Market Report 2025 provides strategists, marketers and senior management with the critical information they need to assess the market.This report focuses on clinical trial supplies market which is experiencing strong growth. The report gives a guide to the trends which will be shaping the market over the next ten years and beyond.
Reasons to Purchase:
- Gain a truly global perspective with the most comprehensive report available on this market covering 15 geographies.
- Assess the impact of key macro factors such as conflict, pandemic and recovery, inflation and interest rate environment and the 2nd Trump presidency.
- Create regional and country strategies on the basis of local data and analysis.
- Identify growth segments for investment.
- Outperform competitors using forecast data and the drivers and trends shaping the market.
- Understand customers based on the latest market shares.
- Benchmark performance against key competitors.
- Suitable for supporting your internal and external presentations with reliable high quality data and analysis
- Report will be updated with the latest data and delivered to you along with an Excel data sheet for easy data extraction and analysis.
- All data from the report will also be delivered in an excel dashboard format.
Description
Where is the largest and fastest growing market for clinical trial supplies? How does the market relate to the overall economy, demography and other similar markets? What forces will shape the market going forward? The clinical trial supplies market global report answers all these questions and many more.The report covers market characteristics, size and growth, segmentation, regional and country breakdowns, competitive landscape, market shares, trends and strategies for this market. It traces the market’s historic and forecast market growth by geography.
- The market characteristics section of the report defines and explains the market.
- The market size section gives the market size ($b) covering both the historic growth of the market, and forecasting its development.
- The forecasts are made after considering the major factors currently impacting the market. These include:
- The forecasts are made after considering the major factors currently impacting the market. These include the Russia-Ukraine war, rising inflation, higher interest rates, and the legacy of the COVID-19 pandemic.
- Market segmentations break down the market into sub markets.
- The regional and country breakdowns section gives an analysis of the market in each geography and the size of the market by geography and compares their historic and forecast growth. It covers the growth trajectory of COVID-19 for all regions, key developed countries and major emerging markets.
- The competitive landscape chapter gives a description of the competitive nature of the market, market shares, and a description of the leading companies. Key financial deals which have shaped the market in recent years are identified.
- The trends and strategies section analyses the shape of the market as it emerges from the crisis and suggests how companies can grow as the market recovers.
Scope
Markets Covered:
1) By Services: Logistics and Distribution; Storage and Retention; Supply Chain Management; Packaging, Labeling, and Blinding; Manufacturing; Comparator Sourcing2) By Clinical Phases: Phase I; Phase II; Phase III; Phase IV
3) By Therapeutic Use: Oncology; Central Nervous System (CNS); Cardiovascular; Infectious Disease; Metabolic Disorders; Other Therapeutic Uses
4) By End User: Pharmaceutical and Biotech companies; Contract Research Organization (CRO); Medical Device Companies
Subsegments:
1) By Logistics and Distribution: Transportation; Inventory Management; Temperature-Controlled Shipping; Customs Clearance2) By Storage and Retention: Short-term Storage; Long-term Storage; Controlled Environment Storage
3) By Supply Chain Management: Planning and Forecasting; Supplier Management; Risk Management; Compliance Management
4) By Packaging, Labeling, and Blinding: Primary Packaging; Secondary Packaging; Label Design and Printing; Blinding Techniques
5) By Manufacturing: Active Pharmaceutical Ingredient (API) Production; Formulation Development; Batch Production
6) By Comparator Sourcing: Sourcing of Reference Products; Quality Control and Assurance; Regulatory Compliance For Comparators
Key Companies Mentioned: Thermo Fisher Scientific Inc.; IQVIA; Eurofins Scientific SE; Parexel International Corporation; ICON PLC
Countries: Australia; Brazil; China; France; Germany; India; Indonesia; Japan; Russia; South Korea; UK; USA; Canada; Italy; Spain
Regions: Asia-Pacific; Western Europe; Eastern Europe; North America; South America; Middle East; Africa
Time Series: Five years historic and ten years forecast.
Data: Ratios of market size and growth to related markets, GDP proportions, expenditure per capita.
Data Segmentation: Country and regional historic and forecast data, market share of competitors, market segments.
Sourcing and Referencing: Data and analysis throughout the report is sourced using end notes.
Delivery Format: PDF, Word and Excel Data Dashboard.
Companies Mentioned
- Thermo Fisher Scientific Inc.
- IQVIA
- Eurofins Scientific SE
- Parexel International Corporation
- ICON PLC
- Catalent Inc.
- Intertek Group PLC
- Recipharm AB
- World Courier
- Almac Group Ltd.
- Piramal Pharma Solutions
- Clinigen Group PLC
- Movianto GmbH
- Marken Limited
- PCI Pharma Services
- Rubicon Research Private Limited
- Bionical Ltd.
- Durbin PLC
- SIRO Clinpharm Pvt. Ltd.
- Biocair International Ltd.
- Ancillare LP.
- Myonex
- Klifo A/S
- Alium Medical Limited
- ADAllen Pharma
- Sharp Services LLC
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 200 |
| Published | February 2025 |
| Forecast Period | 2025 - 2029 |
| Estimated Market Value ( USD | $ 2.88 Billion |
| Forecasted Market Value ( USD | $ 4.03 Billion |
| Compound Annual Growth Rate | 8.7% |
| Regions Covered | Global |
| No. of Companies Mentioned | 26 |