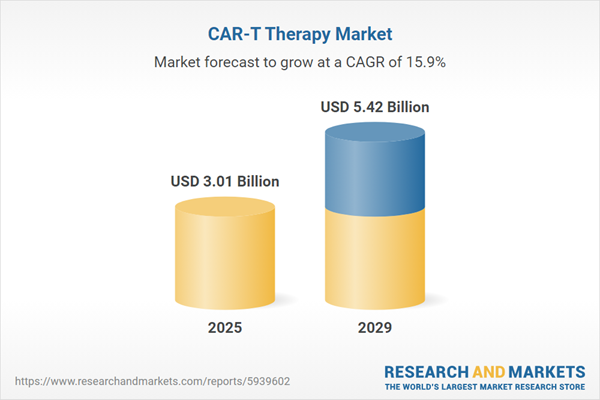
The CAR-T therapy market size is expected to see rapid growth in the next few years. It will grow to $5.42 billion in 2029 at a compound annual growth rate (CAGR) of 15.9%. The growth in the forecast period can be attributed to an increase in blood cancer incidence rate, rise in healthcare expenditure, strong pipeline of drugs, and rising focus on CAR-T therapy. Major trends in the forecast period include investing in the R&D for creating remodeled CAR-T therapy to avoid neurological side effects, creating off-the-shelf allogeneic CAR-T therapy for advanced cancer treatment, manufacturing next-generation CAR T cells for improved treatment of high-grade glioma, investing in ai and machine learning solutions to optimize future CAR-T therapy, carrying out strategic collaborations to boost innovations, collaborating or acquiring competitor companies to expand CAR-T therapy portfolio, and investing in the CAR-T therapy to make it more effective.
The CAR-T therapy market is fueled by the growing financial support from various organizations aimed at promoting the development and utilization of CAR-T therapy. Both governmental and non-governmental organizations provide financial assistance to companies in the CAR-T therapy sector for research and development, as well as to patients undergoing treatment for acute lymphoblastic leukemia (ALL). For example, the Centers for Medicare and Medicaid Services, a U.S.-based federal agency, reported that U.S. health care spending increased by 4.1% to reach $4.5 trillion in 2022, surpassing the 3.2% rise seen in 2021. Furthermore, spending on hospital care services rose by 2.2% in 2022, totaling $1.4 trillion. The financial backing from various organizations positively influences the growth of the CAR-T therapy market.
The escalating prevalence of cancer is anticipated to accelerate the growth of the CAR-T therapy market in the future. The rising prevalence of cancer indicates a growing number of diagnosed cancer cases within a specific population or region over time. This increase has prompted research and investment in cancer therapies, including cellular immunotherapies. These treatments mark a significant advancement in oncology and are especially important for meeting the needs of cancer patients. For instance, in July 2024, the Australian Institute of Health and Welfare, an Australian government agency, reported that cancer diagnoses in Australia rose from 160,570 in 2022 to 164,694 in 2023, reflecting a notable increase over the year. This trend underscores the growing prevalence of cancer nationwide. As a result, the rising incidence of cancer is driving the growth of the CAR-T therapy market.
Key players in the CAR-T therapy market are concentrating on the development of advanced technological solutions, including diagnostic tools, to enhance treatment personalization and improve patient outcomes. Diagnostic tools refer to instruments, devices, or software utilized to identify and assess diseases, conditions, or health issues in individuals. These tools may encompass tests, imaging equipment, or laboratory analyses that offer insights into a patient’s health status, facilitating accurate diagnoses and effective treatment planning. For example, in February 2023, Eurofins Viracor, a U.S.-based clinical diagnostic laboratory, introduced the ExPeCT Expansion and Persistence of CAR T Assay to assist clinicians in gaining a better understanding of the performance of CAR-T therapy for their patients. This assay provides significant advantages for monitoring patients receiving CAR T cell therapy. It allows for longitudinal tracking of CAR T cell expansion and persistence, delivering real-time data that can inform treatment efficacy and patient management. Furthermore, the assay aids in identifying patients at risk of treatment failure due to insufficient CAR T cell dynamics, enabling timely interventions that could enhance clinical outcomes in hematological malignancies.
Major companies operating in the CAR-T cell therapies market are dedicated to innovating new products such as BioCentriq to augment their profitability within the industry. BioCentriq serves as a valuable resource for organizations involved in the realm of cell and gene therapies, facilitating advancements in development, manufacturing, and research efforts, ultimately aiming to enhance patient care. For instance, in October 2023, Terumo Blood and Cell Technologies, a Japanese medical technology company, introduced BioCentriq with the objective of generating accessible public data concerning CAR-T cell manufacturing using Terumo BCT’s CGT (Cell and Gene Therapy) manufacturing platforms. In collaboration with Terumo BCT, BioCentriq strives to pioneer publicly available data focusing on swift CAR-T cell manufacturing, employing the Quantum Flex and Finia platforms. This innovative trial aims to demonstrate the efficient production of high-quality CAR-T cells, leveraging the seamless integration of Terumo BCT's technology and BioCentriq's process expertise to establish streamlined, GMP-translatable workflows.
In July 2023, Astellas Pharma Inc., a Japanese pharmaceutical company, successfully acquired Iveric Bio for $5.9 billion. This strategic move enhances Astellas' ability to address patients at high risk of vision loss due to ocular illnesses. The acquisition leverages Iveric Bio's expertise in ophthalmology alongside Astellas' global resources, aiming to create a leading organization in the field. The transaction was subject to standard closing requirements, including approval from Iveric Bio's stockholders and regulatory approvals. Iveric Bio is a U.S.-based biotech company.
Major companies operating in the CAR-T therapy market include Gilead Sciences, Novartis AG, Biocon, Gracell Biotechnology Ltd, Aeon Therapeutics, CARsgen Therapeutics, Daiichi Sankyo, Otsuka Pharmaceutical, Ono Pharmaceutical, Takeda Pharmaceutical Company, Astellas Pharma, Innovative Cellular Therapeutics, Cellular Biomedicine Group, PersonGen Biomedicine, Hebei Senlang Biotech, Adaptimmune(UK), Autolus Therapeutics, BioNTech, Cellectis, Celyad, Crescendo Biologics, GammaDelta Therapeutics, Immatics, Biocad, Celgene Corporation, Johnson & Johnson, F. Hoffmann-La Roche Ltd, Eli Lilly and Company, Amgen, AstraZeneca, Bayer AG, Merck, Pfizer, Juno Therapeutics, Allogene Therapeutics, Regen BioPharma, OncoSec Medical Incorporated, Precision BioSciences, Bellicum, Sandoz AG, GlaxoSmithKline plc.
North America was the largest region in the CAR-T therapy market in 2024. Western Europe was the second-largest region in the global CAR-T therapy market share. The regions covered in the CAR-T Therapy market report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East, Africa. The countries covered in the CAR-T Therapy market report are Australia, Brazil, China, France, Germany, India, Indonesia, Japan, Russia, South Korea, UK, USA, Italy, Canada, Spain.
Chimeric Antigen Receptor-T (CAR-T) therapy is a form of immunotherapy where T-cells are extracted from the patient's blood, genetically modified in a lab by adding a specialized protein receptor (CAR), enabling the T-cells to recognize and eliminate cancer cells. These modified cells are then infused back into the patient, acting as living drugs in the body.
The primary types of CAR-T therapy are monotherapy and combination therapy. Monotherapy involves treating a disease with a single drug. The targeted antigens include CD19, CD22, and others, applied in various medical conditions such as acute lymphoblastic leukemia, diffuse large B-cell lymphoma, follicular lymphoma, chronic lymphocytic leukemia, multiple myeloma, among others.
The chimeric antigen receptor -T therapy market research report is one of a series of new reports that provides chimeric antigen receptor -T therapy market statistics, including chimeric antigen receptor -T therapy industry global market size, regional shares, competitors with a chimeric antigen receptor -T therapy market share, detailed chimeric antigen receptor -T therapy market segments, market trends and opportunities, and any further data you may need to thrive in the chimeric antigen receptor -T therapy industry. This chimeric antigen receptor -T therapy market research report delivers a complete perspective of everything you need, with an in-depth analysis of the current and future scenario of the industry.
The chimeric antigen receptor -T therapy market includes revenues earned by CAR-T therapy products and related services. The market value includes the value of related goods sold by the service provider or included within the service offering. Only goods and services traded between entities or sold to end consumers are included.
The market value is defined as the revenues that enterprises gain from the sale of goods and/or services within the specified market and geography through sales, grants, or donations in terms of the currency (in USD, unless otherwise specified).
The revenues for a specified geography are consumption values that are revenues generated by organizations in the specified geography within the market, irrespective of where they are produced. It does not include revenues from resales along the supply chain, either further along the supply chain or as part of other products.
This product will be delivered within 3-5 business days.
Table of Contents
Executive Summary
CAR-T Therapy Global Market Report 2025 provides strategists, marketers and senior management with the critical information they need to assess the market.This report focuses on car-t therapy market which is experiencing strong growth. The report gives a guide to the trends which will be shaping the market over the next ten years and beyond.
Reasons to Purchase:
- Gain a truly global perspective with the most comprehensive report available on this market covering 15 geographies.
- Assess the impact of key macro factors such as conflict, pandemic and recovery, inflation and interest rate environment and the 2nd Trump presidency.
- Create regional and country strategies on the basis of local data and analysis.
- Identify growth segments for investment.
- Outperform competitors using forecast data and the drivers and trends shaping the market.
- Understand customers based on the latest market shares.
- Benchmark performance against key competitors.
- Suitable for supporting your internal and external presentations with reliable high quality data and analysis
- Report will be updated with the latest data and delivered to you along with an Excel data sheet for easy data extraction and analysis.
- All data from the report will also be delivered in an excel dashboard format.
Description
Where is the largest and fastest growing market for car-t therapy? How does the market relate to the overall economy, demography and other similar markets? What forces will shape the market going forward? The car-t therapy market global report answers all these questions and many more.The report covers market characteristics, size and growth, segmentation, regional and country breakdowns, competitive landscape, market shares, trends and strategies for this market. It traces the market’s historic and forecast market growth by geography.
- The market characteristics section of the report defines and explains the market.
- The market size section gives the market size ($b) covering both the historic growth of the market, and forecasting its development.
- The forecasts are made after considering the major factors currently impacting the market. These include:
- The forecasts are made after considering the major factors currently impacting the market. These include the Russia-Ukraine war, rising inflation, higher interest rates, and the legacy of the COVID-19 pandemic.
- Market segmentations break down the market into sub markets.
- The regional and country breakdowns section gives an analysis of the market in each geography and the size of the market by geography and compares their historic and forecast growth. It covers the growth trajectory of COVID-19 for all regions, key developed countries and major emerging markets.
- The competitive landscape chapter gives a description of the competitive nature of the market, market shares, and a description of the leading companies. Key financial deals which have shaped the market in recent years are identified.
- The trends and strategies section analyses the shape of the market as it emerges from the crisis and suggests how companies can grow as the market recovers.
Scope
Markets Covered:
1) By Type: Monotherapy, Combination Therapy2) By Target Antigen: CD19, CD22, BCMA, Other Target Antigens
3) By Application: Acute Lymphoblastic Leukemia, Diffuse Large B-Cell Lymphoma, Follicular Lymphoma, Chronic Lymphocytic Leukemia, Multiple Myeloma, Other Applications
Subsegments:
1) By Monotherapy: CD19-targeted CAR-T Therapy; BCMA-Targeted CAR-T Therapy2) By Combination Therapy: CAR-T Therapy With Immune Checkpoint Inhibitors; CAR-T Therapy With Other Targeted Therapies; CAR-T Therapy With Chemotherapy or Radiotherapy
Key Companies Mentioned: Gilead Sciences; Novartis AG; Biocon; Gracell Biotechnology Ltd; Aeon Therapeutics
Countries: Australia; Brazil; China; France; Germany; India; Indonesia; Japan; Russia; South Korea; UK; USA; Canada; Italy; Spain
Regions: Asia-Pacific; Western Europe; Eastern Europe; North America; South America; Middle East; Africa
Time Series: Five years historic and ten years forecast.
Data: Ratios of market size and growth to related markets, GDP proportions, expenditure per capita.
Data Segmentation: Country and regional historic and forecast data, market share of competitors, market segments.
Sourcing and Referencing: Data and analysis throughout the report is sourced using end notes.
Delivery Format: PDF, Word and Excel Data Dashboard.
Companies Mentioned
- Gilead Sciences
- Novartis AG
- Biocon
- Gracell Biotechnology Ltd
- Aeon Therapeutics
- CARsgen Therapeutics
- Daiichi Sankyo
- Otsuka Pharmaceutical
- Ono Pharmaceutical
- Takeda Pharmaceutical Company
- Astellas Pharma
- Innovative Cellular Therapeutics
- Cellular Biomedicine Group
- PersonGen Biomedicine
- Hebei Senlang Biotech
- Adaptimmune(UK)
- Autolus Therapeutics
- BioNTech
- Cellectis
- Celyad
- Crescendo Biologics
- GammaDelta Therapeutics
- Immatics
- Biocad
- Celgene Corporation
- Johnson & Johnson
- F. Hoffmann-La Roche Ltd
- Eli Lilly and Company
- Amgen
- AstraZeneca
- Bayer AG
- Merck
- Pfizer
- Juno Therapeutics
- Allogene Therapeutics
- Regen BioPharma
- OncoSec Medical Incorporated
- Precision BioSciences
- Bellicum
- Sandoz AG
- GlaxoSmithKline plc
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 175 |
| Published | March 2025 |
| Forecast Period | 2025 - 2029 |
| Estimated Market Value ( USD | $ 3.01 Billion |
| Forecasted Market Value ( USD | $ 5.42 Billion |
| Compound Annual Growth Rate | 15.9% |
| Regions Covered | Global |
| No. of Companies Mentioned | 41 |