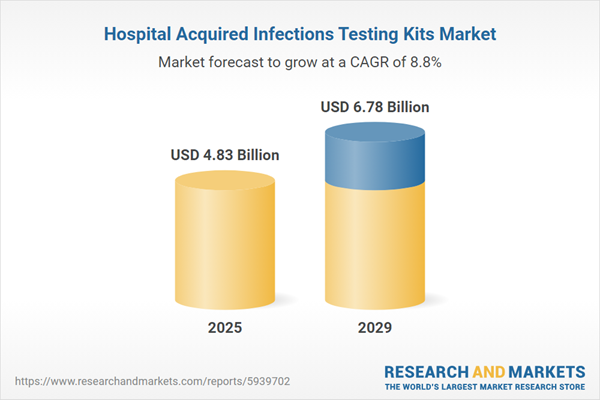
The hospital acquired infections testing kits market size is expected to see strong growth in the next few years. It will grow to $6.78 billion in 2029 at a compound annual growth rate (CAGR) of 8.8%. The growth in the forecast period can be attributed to the increase in chronic diseases, a rise in healthcare expenditure and increasing government support will drive the growth. Major trends in the forecast period include collaborating with artificial intelligence companies, leveraging aid from government, incorporating big data into their market analysis and partnering with other players in the market.
The incidence of hospital-acquired infections (HAIs) is on the rise, impacting both inpatients and outpatients. Hospital-acquired infections, or nosocomial infections, refer to diseases acquired in hospitals and medical clinics. For example, according to the Center for Disease Control and Prevention findings, approximately 1.7 million people in the USA acquire hospital-acquired infections while being admitted for other health issues, with over 98,000 patients (around 5%) succumbing to these infections. The increasing prevalence of hospital-acquired infections contributes to a growing demand for hospital-acquired infections testing kits in the market.
The increasing number of healthcare settings is expected to drive the growth of the hospital-acquired infections testing kits market in the future. Within a healthcare setting, diverse healthcare facilities and services are available, including acute care hospitals, urgent care centers, rehabilitation facilities, nursing homes, and long-term care facilities, collectively providing comprehensive healthcare solutions. Hospital-acquired infections testing kits play a crucial role in detecting and preventing nosocomial infections, thereby enhancing patient safety in healthcare facilities. For instance, in May 2023, according to the American Hospital Association, the total number of hospitals in the U.S. region saw a significant increase from 6,093 in 2022 to 60,129 in 2023. Additionally, admissions in community hospitals also experienced growth, rising from 31,393,318 in 2022 to 31,967,073 in 2023. Therefore, the increasing number of healthcare settings propels the hospital-acquired infections testing kits market.
Advancements in diagnostic tests for diagnosing Hospital-Acquired Infections (HAIs), such as flow cytometry, mass spectrometry, near-infrared spectroscopy, and nucleic acid-based technologies, are enhancing accuracy and providing more precise data. Mass spectrometers, in particular, have seen significant progress, quantified known materials and identified unknown compounds within a sample. For instance, SCIEX's X500 QTOF addresses contamination challenges, offering a compact device without compromising resolution and accuracy. It provides quicker results, enabling operators to make data-driven decisions promptly.
Government agencies, such as the US FDA (Food and Drug Administration) in the USA and the European Medicines Agency/Committee in the European Region, regulate hospital-acquired infections testing kits. These regulatory bodies establish guidelines concerning the safety, efficiency, and efficacy of the instruments within the hospital-acquired infection testing kits market. Manufacturers and service providers must adhere to the CBER Regulations published in the first chapter of Title 21 of CFR. Part 600 of CFR 21 focuses on established standards, including sample retention conditions, required shipment temperatures, equipment sterilization, and the responsibilities of inspectors in laboratory inspections. The regulation also allows manufacturers to report any deviations of their product from its intended purpose. CBER operates under the FDA (Food and Drug Administration). Additionally, the Code of Federal Regulations, Title 21, Part 866, outlines regulations related to immunology and microbiology devices, used for identifying infections caused by bacteria and various viruses.
Major companies operating in the hospital acquired infections testing kits market include Becton, Dickinson and Company, Abbott Laboratories, Cepheid, F. Hoffmann-La Roche Ltd, Hologic, Inc, STERIS plc, QIAGEN, Meridian Bioscience Inc., BioMérieux SA, Metall Zug (Belimed Group), Innovaccer, JD Health, CISCO Systems Inc, GE Healthcare, Honeywell Lifesciences, Zydus Cadila, ImmunoScience, CPC Diagnostics, Seegene, SD Biosensor, Vivacheck Lab, Fujirebio, Sysmex Corporation, Philip Healthcare, Getein Biotech, Hangzhou Biotest Biotech, AmonMed Biotechnology, Beijing Tigsun Diagnostics, Hunan Lituo Biotechnology, Bayer AG, Cantel Medical Corporation, Siemens Healthineers.
North America was the largest region in the hospital-acquired infections testing kits market in 2024. Western Europe was the second largest region in the global hospital-acquired infections testing kits market share. The regions covered in the hospital acquired infections testing kits market report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East, Africa. The countries covered in the hospital acquired infections testing kits market report are Australia, Brazil, China, France, Germany, India, Indonesia, Japan, Russia, South Korea, UK, USA, Italy, Canada, Spain.
The hospital-acquired infections testing kits market consist of sales of testing kits and devices. Values in this market are factory gate values, that is the value of goods sold by the manufacturers or creators of the goods, whether to other entities (including downstream manufacturers, wholesalers, distributors, and retailers) or directly to end customers. The value of goods in this market includes related services sold by the creators of the goods.
Hospital-acquired infection testing kits are utilized to examine infections contracted by individuals within a healthcare facility. These kits play a crucial role in identifying infections associated with pathogens such as vancomycin-resistant enterococci (VRE), methicillin-resistant Staphylococcus aureus (MRSA), multi-resistant gram-negative bacilli, norovirus, and Clostridium difficile.
The primary components of hospital-acquired infection testing kits include instruments, reagents, and consumables. Instruments for these testing kits encompass various tools essential for hospital-acquired infections testing, aiding scientists in cultivating new cells in culture media for purposes such as drug development, tissue culture, engineering, gene therapy, vaccine production, and toxicity testing. Hospital-acquired infections testing kits address pathogens such as viruses, bacteria, and fungi through sterilization, chemical treatments, and radiation methods. The testing extends to identifying infections such as pneumonia, urinary tract infection, bloodstream infection, surgical site infection, MRSA infection, and others. These kits find applications in drug-resistance testing and disease testing.
The hospital-acquired infections testing kits market research report is one of a series of new reports that provides hospital-acquired infections testing kits market statistics, including hospital-acquired infections testing kits industry global market size, regional shares, competitors with a hospital-acquired infections testing kits market share, detailed hospital-acquired infections testing kits market segments, hospital-acquired infections testing kits market trends and opportunities, and any further data you may need to thrive in the hospital-acquired infections testing kits industry. This hospital-acquired infections testing kits market research report delivers a complete perspective of everything you need, with an in-depth analysis of the current and future scenario of industry.
The market value is defined as the revenues that enterprises gain from the sale of goods and/or services within the specified market and geography through sales, grants, or donations in terms of the currency (in USD, unless otherwise specified).
The revenues for a specified geography are consumption values that are revenues generated by organizations in the specified geography within the market, irrespective of where they are produced. It does not include revenues from resales along the supply chain, either further along the supply chain or as part of other products.
This product will be delivered within 3-5 business days.
Table of Contents
Executive Summary
Hospital Acquired Infections Testing Kits Global Market Report 2025 provides strategists, marketers and senior management with the critical information they need to assess the market.This report focuses on hospital acquired infections testing kits market which is experiencing strong growth. The report gives a guide to the trends which will be shaping the market over the next ten years and beyond.
Reasons to Purchase:
- Gain a truly global perspective with the most comprehensive report available on this market covering 15 geographies.
- Assess the impact of key macro factors such as conflict, pandemic and recovery, inflation and interest rate environment and the 2nd Trump presidency.
- Create regional and country strategies on the basis of local data and analysis.
- Identify growth segments for investment.
- Outperform competitors using forecast data and the drivers and trends shaping the market.
- Understand customers based on the latest market shares.
- Benchmark performance against key competitors.
- Suitable for supporting your internal and external presentations with reliable high quality data and analysis
- Report will be updated with the latest data and delivered to you along with an Excel data sheet for easy data extraction and analysis.
- All data from the report will also be delivered in an excel dashboard format.
Description
Where is the largest and fastest growing market for hospital acquired infections testing kits? How does the market relate to the overall economy, demography and other similar markets? What forces will shape the market going forward? The hospital acquired infections testing kits market global report answers all these questions and many more.The report covers market characteristics, size and growth, segmentation, regional and country breakdowns, competitive landscape, market shares, trends and strategies for this market. It traces the market’s historic and forecast market growth by geography.
- The market characteristics section of the report defines and explains the market.
- The market size section gives the market size ($b) covering both the historic growth of the market, and forecasting its development.
- The forecasts are made after considering the major factors currently impacting the market. These include:
- The forecasts are made after considering the major factors currently impacting the market. These include the Russia-Ukraine war, rising inflation, higher interest rates, and the legacy of the COVID-19 pandemic.
- Market segmentations break down the market into sub markets.
- The regional and country breakdowns section gives an analysis of the market in each geography and the size of the market by geography and compares their historic and forecast growth. It covers the growth trajectory of COVID-19 for all regions, key developed countries and major emerging markets.
- The competitive landscape chapter gives a description of the competitive nature of the market, market shares, and a description of the leading companies. Key financial deals which have shaped the market in recent years are identified.
- The trends and strategies section analyses the shape of the market as it emerges from the crisis and suggests how companies can grow as the market recovers.
Scope
Markets Covered:
1) By Product: Consumables and Reagents, Instruments2) By Test Type: Pneumoia Infection, Urinary Track Infection, Blood Stream Associated Infection, Surgical Site Infection, MRSA infection, Others
3) By Pathogen Type: Viral, Bacterial, Fungal
4) By Application: Drug Resistance Testing, Disease Testing
Subsegments:
1) By Consumables and Reagents: Culture Media (Used for isolating and identifying pathogens); Reagents For Molecular Testing (Includes PCR reagents and kits for detecting specific pathogens); Test Kits (Rapid tests and other kits designed for various infections); Other Consumables (Includes swabs, sample collection devices, and preservation solutions)2) By Instruments: Automated Analyzers (Equipment used for blood culture analysis and identification of pathogens); Molecular Diagnostic Instruments (Machines used for PCR and other molecular testing methods); Microscopes (Used for the identification of pathogens in samples); Incubators and Growth Chambers (For culturing samples under controlled conditions)
Key Companies Mentioned: Becton, Dickinson and Company; Abbott Laboratories; Cepheid; F. Hoffmann-La Roche Ltd; Hologic, Inc
Countries: Australia; Brazil; China; France; Germany; India; Indonesia; Japan; Russia; South Korea; UK; USA; Canada; Italy; Spain
Regions: Asia-Pacific; Western Europe; Eastern Europe; North America; South America; Middle East; Africa
Time Series: Five years historic and ten years forecast.
Data: Ratios of market size and growth to related markets, GDP proportions, expenditure per capita.
Data Segmentation: Country and regional historic and forecast data, market share of competitors, market segments.
Sourcing and Referencing: Data and analysis throughout the report is sourced using end notes.
Delivery Format: PDF, Word and Excel Data Dashboard.
Companies Mentioned
- Becton, Dickinson and Company
- Abbott Laboratories
- Cepheid
- F. Hoffmann-La Roche Ltd
- Hologic, Inc
- STERIS plc
- QIAGEN
- Meridian Bioscience Inc.
- BioMérieux SA
- Metall Zug (Belimed Group)
- Innovaccer
- JD Health
- CISCO Systems Inc
- GE Healthcare
- Honeywell Lifesciences
- Zydus Cadila
- ImmunoScience
- CPC Diagnostics
- Seegene
- SD Biosensor
- Vivacheck Lab
- Fujirebio
- Sysmex Corporation
- Philip Healthcare
- Getein Biotech
- Hangzhou Biotest Biotech
- AmonMed Biotechnology
- Beijing Tigsun Diagnostics
- Hunan Lituo Biotechnology
- Bayer AG
- Cantel Medical Corporation
- Siemens Healthineers
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 250 |
| Published | March 2025 |
| Forecast Period | 2025 - 2029 |
| Estimated Market Value ( USD | $ 4.83 Billion |
| Forecasted Market Value ( USD | $ 6.78 Billion |
| Compound Annual Growth Rate | 8.8% |
| Regions Covered | Global |
| No. of Companies Mentioned | 32 |