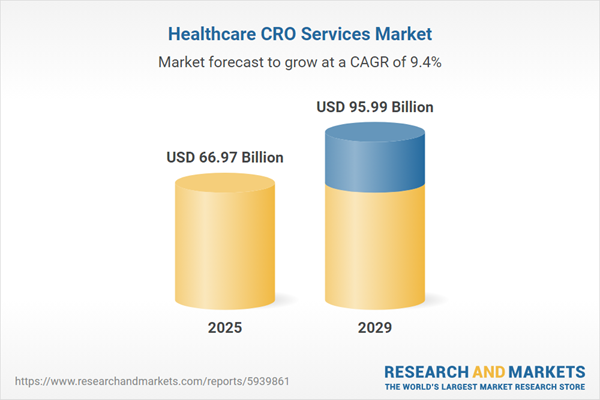
The healthcare CRO services market size is expected to see strong growth in the next few years. It will grow to $95.99 billion in 2029 at a compound annual growth rate (CAGR) of 9.4%. The growth in the forecast period can be attributed to rise of biologics and cell and gene therapies, increased focus on rare diseases, focus on cost-effectiveness and speed, growing demand for real-world data, focus on patient-centricity, biopharmaceutical pipeline growth. Major trends in the forecast period include integration of artificial intelligence (ai), integration of machine learning (ml), environmental, social, and governance (ESG) considerations, rising R&D investments by pharmaceutical companies.
The growth of the healthcare CRO services market is being driven by the increase in healthcare expenditures. Healthcare expenditures encompass all costs related to providing health services, family planning, nutrition programs, and emergency health assistance. As healthcare spending rises, it enables a larger pool of funding for medical research and development, which in turn boosts the demand for healthcare CRO services, particularly in supporting clinical trials and studies. For example, in 2022, the Office for National Statistics, a UK-based national statistics organization, reported that healthcare spending in the UK reached approximately $354.88 billion (£283 billion), marking a nominal increase of 0.7% compared to 2021. Consequently, the rise in healthcare spending is contributing to the expansion of the healthcare CRO services market.
The increasing number of clinical trials is expected to further boost the growth of the healthcare CRO services market. Clinical trials are scientific studies involving human participants designed to assess the safety and effectiveness of new medical interventions, such as drugs, therapies, devices, or procedures. Contract research organizations (CROs) play a vital role in the clinical trial process by providing various services that assist pharmaceutical and biotechnology companies in designing, conducting, and managing their studies efficiently. For instance, according to a report from Xtalks, a Canada-based web news and information network, there were 452,604 clinical studies registered on ClinicalTrials.gov as of May 2023, representing a significant increase from the over 365,000 registered trials recorded in early 2021. Therefore, the growth in clinical trials is contributing to the expansion of the healthcare CRO services market.
Major players in the healthcare CRO services market are adopting a strategic partnership approach to provide advanced Proteomics CRO services to biopharmaceutical and biomarker clients on a global scale. Strategic partnerships involve companies leveraging each other's strengths and resources for mutual benefits and success. In January 2023, Bruker Corporation, a US-based manufacturer of scientific instruments, formed a partnership with Biognosys AG, a Switzerland-based healthcare CRO services provider. The collaboration between Bruker and Biognosys is expected to create unique synergies, combining Biognosys' extensive range of proprietary proteomics services, software, and kits with Bruker's innovative timsTOF platform. This strategic partnership will result in the establishment of Biognosys' inaugural laboratory for advanced proteomics CRO services in the United States.
Major companies in the healthcare CRO services market are also innovating by developing products such as clinical trial monitoring solutions to expand their customer bases, boost sales, and increase revenue. A clinical trial monitoring solution is a comprehensive system or set of tools designed to oversee and manage various aspects of clinical trials. For example, in April 2022, Tata Consultancy Services (TCS), an India-based information technology (IT) services and consulting company, introduced a Risk-Based Monitoring solution. This innovative solution utilizes advanced statistical algorithms to proactively identify and address study and site risks in biopharmaceutical and Contract Research Organizations (CROs). Using data science, the solution accurately predicts outcomes related to site workload and risks, enabling stakeholders to implement informed monitoring strategies. The Risk-Based Monitoring solution provides unprecedented insights into missing data, enhancing data quality, consistency, and early detection of trends. With the potential for up to a 30% efficiency gain in site monitoring, the solution's automation streamlines processes, reduces site workload, ensures compliance, and accelerates product speed-to-market.
In May 2023, Elliott Investment Management L.P., a US-based investment management firm, along with Patient Square Capital, a US-based capital market company, and Veritas Capital Fund Management LLC, a US-based quantitative and systematic asset management firm, acquired Syneos Health Inc. for $7.1 billion. Through this acquisition, the consortium of private investment firms seeks to enhance its portfolio by leveraging Syneos Health's expertise in healthcare contract research organization (CRO) services. Syneos Health Inc. is a US-based company specializing in providing CRO services to the healthcare industry.
Major companies operating in the healthcare CRO services market include Laboratory Corporation of America Holdings., IQVIA Inc., Icon Plc., PPD Inc., Wuxi Apptec Co. Ltd., Syneos Health Inc., Parexel International Corporation, Charles River Laboratories International Inc., Covance Inc., Medpace Inc., Pharmaron Beijing Co. Ltd., BioClinica Inc., Chiltern International Ltd., Evotec SE, PSI CRO AG, Premier Research Group Ltd., Worldwide Clinical Trials Inc., Caidya, BioTelemetry Inc., Syngene International Limited, Novotech CRO, Celerion, GVK Biosciences Private Limited, Synteract Inc., Veristat LLC., ACM Global Laboratories, ClinTec Luxembourg SA, Clinlogix LLC.
North America was the largest region in the healthcare CRO services market in 2024. Asia-Pacific is expected to be the fastest-growing region in the forecast period. The regions covered in the healthcare cro services market report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East, Africa. The countries covered in the healthcare cro services market report are Australia, Brazil, China, France, Germany, India, Indonesia, Japan, Russia, South Korea, UK, USA, Canada, Italy, Spain.
Healthcare Contract Research Organization (CRO) services encompass the outsourced support and specialized expertise provided to the pharmaceutical, biotechnology, and medical device sectors by dedicated businesses. These entities seek assistance from contract research organizations for a range of research-related tasks, including data management, conducting clinical trials, and preparing regulatory submissions.
The primary categories of healthcare CRO services include early-phase development, clinical development services, laboratory services, and consulting services. Early-phase development denotes the initial stage of a project or enterprise, involving the investigation, testing, and validation of theories or hypotheses to establish the foundation for future development and progress. These services find application in various therapeutic areas such as oncology, CNS disorders, cardiovascular diseases, metabolic diseases, infectious diseases, diabetes, and others. Multiple end-users, including pharmaceutical companies, medical device companies, and academic institutes, engage in the utilization of these services.
The healthcare CRO services market research report is one of a series of new reports that provides healthcare CRO services market statistics, including healthcare CRO services industry global market size, regional shares, competitors with a healthcare CRO services market share, detailed healthcare CRO services market segments, market trends and opportunities, and any further data you may need to thrive in the healthcare CRO services industry. This healthcare CRO services market research report delivers a complete perspective of everything you need, with an in-depth analysis of the current and future scenario of the industry.
The healthcare CRO services market includes revenues earned by entities by providing services such as discovery studies, pre-clinical services, analytical testing, and related pharmacovigilance and drug safety, data management, and biostatistics. The market value includes the value of related goods sold by the service provider or included within the service offering. Only goods and services traded between entities or sold to end consumers are included.
The market value is defined as the revenues that enterprises gain from the sale of goods and/or services within the specified market and geography through sales, grants, or donations in terms of the currency (in USD, unless otherwise specified).
The revenues for a specified geography are consumption values that are revenues generated by organizations in the specified geography within the market, irrespective of where they are produced. It does not include revenues from resales along the supply chain, either further along the supply chain or as part of other products.
This product will be delivered within 3-5 business days.
Table of Contents
Executive Summary
Healthcare CRO Services Global Market Report 2025 provides strategists, marketers and senior management with the critical information they need to assess the market.This report focuses on healthcare cro services market which is experiencing strong growth. The report gives a guide to the trends which will be shaping the market over the next ten years and beyond.
Reasons to Purchase:
- Gain a truly global perspective with the most comprehensive report available on this market covering 15 geographies.
- Assess the impact of key macro factors such as conflict, pandemic and recovery, inflation and interest rate environment and the 2nd Trump presidency.
- Create regional and country strategies on the basis of local data and analysis.
- Identify growth segments for investment.
- Outperform competitors using forecast data and the drivers and trends shaping the market.
- Understand customers based on the latest market shares.
- Benchmark performance against key competitors.
- Suitable for supporting your internal and external presentations with reliable high quality data and analysis
- Report will be updated with the latest data and delivered to you along with an Excel data sheet for easy data extraction and analysis.
- All data from the report will also be delivered in an excel dashboard format.
Description
Where is the largest and fastest growing market for healthcare cro services? How does the market relate to the overall economy, demography and other similar markets? What forces will shape the market going forward? The healthcare cro services market global report answers all these questions and many more.The report covers market characteristics, size and growth, segmentation, regional and country breakdowns, competitive landscape, market shares, trends and strategies for this market. It traces the market’s historic and forecast market growth by geography.
- The market characteristics section of the report defines and explains the market.
- The market size section gives the market size ($b) covering both the historic growth of the market, and forecasting its development.
- The forecasts are made after considering the major factors currently impacting the market. These include:
- The forecasts are made after considering the major factors currently impacting the market. These include the Russia-Ukraine war, rising inflation, higher interest rates, and the legacy of the COVID-19 pandemic.
- Market segmentations break down the market into sub markets.
- The regional and country breakdowns section gives an analysis of the market in each geography and the size of the market by geography and compares their historic and forecast growth. It covers the growth trajectory of COVID-19 for all regions, key developed countries and major emerging markets.
- The competitive landscape chapter gives a description of the competitive nature of the market, market shares, and a description of the leading companies. Key financial deals which have shaped the market in recent years are identified.
- The trends and strategies section analyses the shape of the market as it emerges from the crisis and suggests how companies can grow as the market recovers.
Scope
Markets Covered:
1) By Service Type: Early Phase Development; Clinical Development Services; Laboratory Services; Consulting Services2) By Therapeutic Area: Oncology; CNS Disorder; Cardiovascular Disease; Metabolic Disease; Infectious Disease; Diabetes; Other Therapeutic Areas
3) By End User: Pharmaceutical Companies; Medical Device Companies; Academic Institutes
Subsegments:
1) By Early Phase Development: Preclinical Studies; Phase I Trials; Formulation Development2) By Clinical Development Services: Phase II Trials; Phase III Trials; Patient Recruitment and Retention; Site Management
3) By Laboratory Services: Bioanalytical Testing; Clinical Laboratory Services; Safety and Toxicology Testing
4) By Consulting Services: Regulatory Affairs Consulting; Market Access and Commercialization; Strategic Planning and Feasibility Studies
Key Companies Mentioned: Laboratory Corporation of America Holdings.; IQVIA Inc.; Icon Plc.; PPD Inc.; Wuxi Apptec Co. Ltd.
Countries: Australia; Brazil; China; France; Germany; India; Indonesia; Japan; Russia; South Korea; UK; USA; Canada; Italy; Spain
Regions: Asia-Pacific; Western Europe; Eastern Europe; North America; South America; Middle East; Africa
Time Series: Five years historic and ten years forecast.
Data: Ratios of market size and growth to related markets, GDP proportions, expenditure per capita.
Data Segmentation: Country and regional historic and forecast data, market share of competitors, market segments.
Sourcing and Referencing: Data and analysis throughout the report is sourced using end notes.
Delivery Format: PDF, Word and Excel Data Dashboard.
Companies Mentioned
- Laboratory Corporation of America Holdings.
- IQVIA Inc.
- Icon Plc.
- PPD Inc.
- Wuxi Apptec Co. Ltd.
- Syneos Health Inc.
- Parexel International Corporation
- Charles River Laboratories International Inc.
- Covance Inc.
- Medpace Inc.
- Pharmaron Beijing Co. Ltd.
- BioClinica Inc.
- Chiltern International Ltd.
- Evotec SE
- PSI CRO AG
- Premier Research Group Ltd.
- Worldwide Clinical Trials Inc.
- Caidya
- BioTelemetry Inc.
- Syngene International Limited
- Novotech CRO
- Celerion
- GVK Biosciences Private Limited
- Synteract Inc.
- Veristat LLC.
- ACM Global Laboratories
- ClinTec Luxembourg SA
- Clinlogix LLC
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 200 |
| Published | February 2025 |
| Forecast Period | 2025 - 2029 |
| Estimated Market Value ( USD | $ 66.97 Billion |
| Forecasted Market Value ( USD | $ 95.99 Billion |
| Compound Annual Growth Rate | 9.4% |
| Regions Covered | Global |
| No. of Companies Mentioned | 28 |