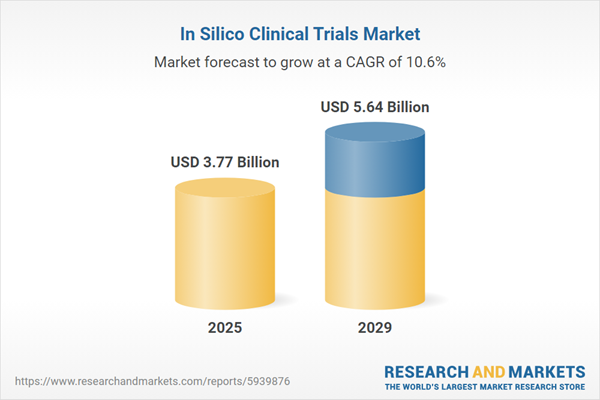
The in silico clinical trials market size is expected to see rapid growth in the next few years. It will grow to $5.64 billion in 2029 at a compound annual growth rate (CAGR) of 10.6%. The growth in the forecast period can be attributed to growing demand for faster drug development processes, adoption of virtual patient populations in clinical trials, expansion of digital twin technologies, increase in regulatory acceptance of in silico trials. Major trends in the forecast period include development of more realistic virtual patient models, integration of real-world evidence in in silico trials, emphasis on decentralized and patient-centric trial designs, rise in the use of digital biomarkers, increased focus on personalized medicine in virtual trials.
The increasing prevalence of infectious diseases is expected to drive growth in the in silico clinical trials market in the future. This rise is influenced by factors such as urbanization, overcrowding, climate change, microbial evolution, and increased travel. In silico clinical trials utilize computational models and simulations to expedite the development of treatments for infectious diseases, allowing researchers to analyze disease mechanisms, predict drug efficacy, and optimize clinical trial designs, ultimately enhancing patient outcomes and advancing public health initiatives. For example, according to gov.uk, a UK-based public sector information website, England recorded 3,805 new HIV diagnoses in 2022, representing a 22% increase from the 3,118 cases reported in 2021 and a 26% rise from the 3,026 cases in 2020. Thus, the growing prevalence of infectious diseases is driving the in silico clinical trials market.
The expansion of the in silico clinical trials market is further fueled by the need to reduce clinical trial costs. Clinical trial costs encompass the expenses associated with planning, conducting, and managing trials to assess the safety and efficacy of medical interventions. Traditional trials are lengthy and expensive, taking 10-15 years and costing billions. In silico trials offer an alternative, using computer simulations to evaluate interventions. For instance, according to Genetic Engineering and Biotechnology News, the cost of developing a new drug among the top 20 global biopharma companies rose from $298 million in 2022 to approximately $2.3 billion in 2023, including clinical trial development costs. This shift towards in silico clinical trials is driven by the growing need to curb these escalating expenses.
A prominent trend in the in silico clinical trials market is the emergence of strategic partnerships. Major companies in the market are forming partnerships to enhance their market position. For instance, in March 2023, Premier Research, a US-based clinical research organization (CRO), partnered with InSilicoTrials to create efficient pathways for rare disease treatment approval. Similarly, 4P-Pharma, a France-based biotech company, collaborated with QuantHealth, an Israel-based AI company, to conduct patient-centric drug simulations, accelerating and de-risking drug development. These partnerships exemplify the industry trend of forming strategic alliances to strengthen market presence and facilitate advancements in in silico clinical trials.
Prominent players in the in silico clinical trials market are directing their focus towards cutting-edge technologies, specifically clinical trial platforms, to uphold their market positions. A clinical trial platform represents a specialized form of an adaptive, disease-centric, randomized clinical trial (RCT) formulated to assess multiple interventions concurrently against a consistent control group. As an illustration, in October 2023, Advarra, a US-based provider offering regulatory review solutions and clinical research technology for sites and sponsors, introduced Longboat v2.2. This new technological functionality aims to enhance the overall clinical trial experiences of stakeholders. Longboat, an established platform utilized in over 70 countries and by nearly 20,000 sites, now incorporates an online patient portal equipped with a comprehensive array of engagement tools. This includes a straightforward document exchange feature facilitating transparent collaboration among patients, sites, sponsors, or clinical research organizations (CROs), all while avoiding an additional technology burden.
In May 2023, Recursion, a US-based clinical-stage TechBio company, disclosed the acquisition of Cyclica and Valence for an undisclosed sum. These strategic acquisitions bring cutting-edge capabilities in digital chemistry, as well as machine learning and artificial intelligence from Cyclica and Valence. These capabilities are seamlessly integrated with Recursion's extensive automated wet-laboratories and supercomputing resources. This integration positions Recursion to deploy the most comprehensive, technology-enabled drug discovery solution within the biopharma industry. Cyclica, a biotechnology company based in Canada, and Valence, a Canadian deep learning research institute, collectively contribute to enhancing Recursion's capabilities in the pursuit of advancing drug discovery through innovative technologies.
Major companies operating in the in silico clinical trials market include Dassault Systemes SE, Clarivate plc, Evotec A.G, Evidera, Certara Inc., Abzena Limited, Selvita, Simulations Plus Inc., Insilico Medicine Inc., AnyLogic Company, Biomax Informatics, GNS Healthcare Inc., 4P-Pharma, Nuventra Pharma Sciences, Archimedes, Novadiscovery Sas, Rosa & Co., In Silico Biosciences, Leadscope, Biognos Ab, BioNova, Immunetrics Inc., InSilicoTrials, Physiomics plc, InhibOx, Entelos.
North America was the largest region in the in silico clinical trials market in 2024. Asia-Pacific is expected to be the fastest-growing region in the in silico clinical trials market report during the forecast period. The regions covered in the in silico clinical trials market report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East, Africa. The countries covered in the in silico clinical trials market report are Australia, Brazil, China, France, Germany, India, Indonesia, Japan, Russia, South Korea, UK, USA, Canada, Italy, Spain.
In silico clinical trials, also known as virtual clinical trials, involve computer simulations used to develop or evaluate the safety and efficacy of medicinal products, devices, or interventions. They serve as a method to assess the safety and effectiveness of medical devices and imaging systems.
The primary phases of in silico clinical trials include Phase I, Phase II, Phase III, and Phase IV. Phase I represents the initial evaluation of a drug or treatment on a small group of people, aiming to assess the safety and tolerability of new drugs in humans. These trials are conducted across various medical domains such as oncology, infectious disease, hematology, cardiology, dermatology, neurology, diabetes, and others. In silico clinical trials find applications in both the medical devices and pharmaceutical industries.
The in silico clinical trials research report is one of a series of new reports that provides in silico clinical trials market statistics, including the in silico clinical trials industry's global market size, regional shares, competitors with in silico clinical trials market share, detailed in silico clinical trials market segments, market trends and opportunities, and any further data you may need to thrive in the in silico clinical trials industry. This in silico clinical trials market research report delivers a complete perspective of everything you need, with an in-depth analysis of the current and future scenarios of the industry.
The in silico clinical trials market includes revenues earned by entities by providing services such as data management, trial design, information compilation and site support. The market value includes the value of related goods sold by the service provider or included within the service offering. Only goods and services traded between entities or sold to end consumers are included.
The market value is defined as the revenues that enterprises gain from the sale of goods and/or services within the specified market and geography through sales, grants, or donations in terms of the currency (in USD, unless otherwise specified).
The revenues for a specified geography are consumption values that are revenues generated by organizations in the specified geography within the market, irrespective of where they are produced. It does not include revenues from resales along the supply chain, either further along the supply chain or as part of other products.
This product will be delivered within 3-5 business days.
Table of Contents
Executive Summary
in Silico Clinical Trials Global Market Report 2025 provides strategists, marketers and senior management with the critical information they need to assess the market.This report focuses on in silico clinical trials market which is experiencing strong growth. The report gives a guide to the trends which will be shaping the market over the next ten years and beyond.
Reasons to Purchase:
- Gain a truly global perspective with the most comprehensive report available on this market covering 15 geographies.
- Assess the impact of key macro factors such as conflict, pandemic and recovery, inflation and interest rate environment and the 2nd Trump presidency.
- Create regional and country strategies on the basis of local data and analysis.
- Identify growth segments for investment.
- Outperform competitors using forecast data and the drivers and trends shaping the market.
- Understand customers based on the latest market shares.
- Benchmark performance against key competitors.
- Suitable for supporting your internal and external presentations with reliable high quality data and analysis
- Report will be updated with the latest data and delivered to you along with an Excel data sheet for easy data extraction and analysis.
- All data from the report will also be delivered in an excel dashboard format.
Description
Where is the largest and fastest growing market for in silico clinical trials? How does the market relate to the overall economy, demography and other similar markets? What forces will shape the market going forward? The in silico clinical trials market global report answers all these questions and many more.The report covers market characteristics, size and growth, segmentation, regional and country breakdowns, competitive landscape, market shares, trends and strategies for this market. It traces the market’s historic and forecast market growth by geography.
- The market characteristics section of the report defines and explains the market.
- The market size section gives the market size ($b) covering both the historic growth of the market, and forecasting its development.
- The forecasts are made after considering the major factors currently impacting the market. These include:
- The forecasts are made after considering the major factors currently impacting the market. These include the Russia-Ukraine war, rising inflation, higher interest rates, and the legacy of the COVID-19 pandemic.
- Market segmentations break down the market into sub markets.
- The regional and country breakdowns section gives an analysis of the market in each geography and the size of the market by geography and compares their historic and forecast growth. It covers the growth trajectory of COVID-19 for all regions, key developed countries and major emerging markets.
- The competitive landscape chapter gives a description of the competitive nature of the market, market shares, and a description of the leading companies. Key financial deals which have shaped the market in recent years are identified.
- The trends and strategies section analyses the shape of the market as it emerges from the crisis and suggests how companies can grow as the market recovers.
Scope
Markets Covered:
1) By Phase: Phase I; Phase II; Phase III; Phase IV2) By Therapeutic Area: Oncology; Infectious Disease; Hematology; Cardiology; Dermatology; Neurology; Diabetes; Other Therapeutic Areas
3) By Industry: Medical Devices; Pharmaceutical
Subsegments:
1) By Phase I: Safety and Dosage Trials; Pharmacokinetics and Pharmacodynamics Studies2) By Phase II: Efficacy Trials; Dose-Response Studies
3) By Phase III: Comparative Effectiveness Trials; Large-Scale Efficacy Studies
4) By Phase IV: Post-Marketing Surveillance; Long-Term Safety Studies
Key Companies Mentioned: Dassault Systemes SE; Clarivate plc; Evotec a.G; Evidera; Certara Inc.
Countries: Australia; Brazil; China; France; Germany; India; Indonesia; Japan; Russia; South Korea; UK; USA; Canada; Italy; Spain
Regions: Asia-Pacific; Western Europe; Eastern Europe; North America; South America; Middle East; Africa
Time Series: Five years historic and ten years forecast.
Data: Ratios of market size and growth to related markets, GDP proportions, expenditure per capita.
Data Segmentation: Country and regional historic and forecast data, market share of competitors, market segments.
Sourcing and Referencing: Data and analysis throughout the report is sourced using end notes.
Delivery Format: PDF, Word and Excel Data Dashboard.
Companies Mentioned
- Dassault Systemes SE
- Clarivate plc
- Evotec A.G
- Evidera
- Certara Inc.
- Abzena Limited
- Selvita
- Simulations Plus Inc.
- Insilico Medicine Inc.
- AnyLogic Company
- Biomax Informatics
- GNS Healthcare Inc.
- 4P-Pharma
- Nuventra Pharma Sciences
- Archimedes
- Novadiscovery Sas
- Rosa & Co.
- In Silico Biosciences
- Leadscope
- Biognos Ab
- BioNova
- Immunetrics Inc.
- InSilicoTrials
- Physiomics plc
- InhibOx
- Entelos
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 200 |
| Published | February 2025 |
| Forecast Period | 2025 - 2029 |
| Estimated Market Value ( USD | $ 3.77 Billion |
| Forecasted Market Value ( USD | $ 5.64 Billion |
| Compound Annual Growth Rate | 10.6% |
| Regions Covered | Global |
| No. of Companies Mentioned | 26 |