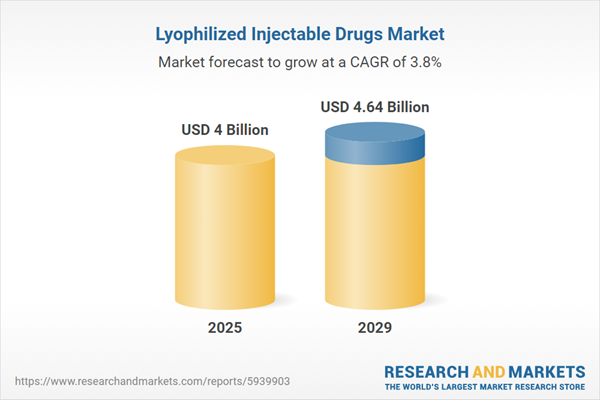
The lyophilized injectable drugs market size is expected to see steady growth in the next few years. It will grow to $4.64 billion in 2029 at a compound annual growth rate (CAGR) of 3.8%. The growth in the forecast period can be attributed to rise in personalized medicine, expansion of biologics pipeline, global health preparedness, demand for orphan drugs, increased focus on patient compliance. Major trends in the forecast period include innovations in lyophilized combination therapies, expansion of lyophilized vaccines, integration of quality-by-design (QBD) principles, utilization of lyophilized drugs in emergency medicine, regulatory emphasis on lyophilization process validation.
The forecast of 3.8% growth over the next five years reflects a slight reduction of 0.1% from the previous projection. This reduction is primarily due to the impact of tariffs between the US and other countries. Trade tensions could hinder critical care medicine by inflating prices of freeze-dried antibiotic and biologic formulations manufactured in South Korea and Denmark, resulting in medication shortages and higher hospital pharmacy costs. The effect will also be felt more widely due to reciprocal tariffs and the negative effect on the global economy and trade due to increased trade tensions and restrictions.
The increasing incidence of cardiac disorders is projected to drive the growth of the lyophilized injectable drugs market in the future. Cardiac disorders encompass a wide range of conditions affecting the heart's structure and function, including issues with the valves, chambers, blood vessels, electrical system, and overall pumping ability. Lyophilized injectable drugs, such as thrombolytic agents, antiarrhythmic medications, inotropic agents, and antihypertensive medications, are utilized in treating various cardiac disorders due to their stability, convenience, flexibility, rapid reconstitution, improved bioavailability, and reduced risk of contamination. For example, data published by the British Heart Foundation, a UK-based cardiovascular research charity, revealed that approximately 7.6 million people in the UK are affected by heart and circulatory diseases, including around 4 million males and 3.6 million females. These conditions account for about 27% of all deaths in the UK, resulting in over 170,000 fatalities annually, or roughly 480 deaths each day - equating to one death every three minutes. Therefore, the rising incidence of cardiac disorders is a significant driver for the growth of the lyophilized injectable drugs market during the forecast period.
The increasing demand within pharmaceutical industries is anticipated to drive the growth of the lyophilized injectable drugs market in the coming years. This growth in the pharmaceutical sector can be attributed to ongoing R&D innovations, successful regulatory approvals, an aging population, technological advancements, globalization, and improved access to healthcare. As the pharmaceutical industry expands, the need for stable, long-lasting drug formulations rises. For example, in February 2023, Merck & Co. Inc., a US-based pharmaceutical company, reported global sales of $59.3 billion for 2022, marking a 22% increase compared to the previous year. Therefore, the rising demand in pharmaceutical industries is a key factor fueling the growth of the lyophilized injectable drugs market.
The escalating incidence of cardiovascular diseases is poised to propel the lyophilized injectable drugs market. Cardiovascular diseases encompass a spectrum of conditions affecting the heart and blood vessels, including coronary artery disease, heart failure, arrhythmias, and heart valve problems. Lyophilized injectable drugs address cardiovascular diseases by providing stable and durable formulations that enhance drug efficacy, ensure precise dosing, and enable convenient administration. This contributes to improved patient outcomes and treatment compliance. In 2022, according to data from the Centers for Disease Control and Prevention (CDC), heart disease accounted for over 659,000 deaths in the United States, representing one in every four fatalities. Specifically, coronary heart disease, the most prevalent type, claimed 375,476 lives in 2021. Therefore, the escalating cases of cardiovascular diseases are expected to drive the lyophilized injectable drugs market forward.
The prevailing trend in the lyophilized injectable drug market is the emphasis on product innovations, with major companies actively pursuing novel drug delivery systems to enhance their market profitability. Notably, in June 2022, Gufic Biosciences Ltd., an India-based pharmaceutical company, introduced Dual Chamber Bags (DCB), a critical innovation for reconstituting lyophilized injectable drugs. These bags offer multiple advantages, including a reduction in the risk of bloodstream infections, ensuring patient safety, and preventing prescription errors.
Leading companies in the lyophilized injectable drug market are channeling their efforts into leveraging innovative technologies, such as non-invasive technology, for the development of lyophilized injectable drugs. Non-invasive technology involves methods or devices that gather information or perform tasks without penetrating the skin or entering bodily orifices. For example, in October 2023, COMSER Pharma, a Spain-based provider of compliance services and freeze-dry pharmaceutical solutions, introduced LyoFlow, an innovative technology for lyophilized injectable drug production. LyoFlow is a disruptive solution enabling real-time, non-invasive extraction of the mass flow in a freeze-drying process, easily integratable into any lyophilization equipment, and facilitating continuous monitoring of product quality throughout the process.
In April 2022, Recipharm AB, a pharmaceutical contract development and manufacturing organization based in Sweden, acquired Vibalogics for an undisclosed amount. This acquisition aims to reinforce the company's position in the biologics market, with a specific focus on manufacturing drug ingredients for novel advanced therapeutic medicinal goods. Vibalogics, a Germany-based manufacturing company, specializes in the production of sophisticated live biological products, operating within the lyophilized injectable drugs market.
Note that the outlook for this market is being affected by rapid changes in trade relations and tariffs globally. The report will be updated prior to delivery to reflect the latest status, including revised forecasts and quantified impact analysis. The report’s Recommendations and Conclusions sections will be updated to give strategies for entities dealing with the fast-moving international environment.
The sudden escalation of U.S. tariffs and the resulting trade tensions in spring 2025 are having a significant impact on the pharmaceutical sector. Companies are grappling with higher costs on imported active pharmaceutical ingredients (APIs), glass vials, and laboratory equipment - many of which have limited alternative sources. Generic drug manufacturers, already operating with minimal profit margins, are particularly affected, with some scaling back production of low-margin medications. Biotech firms are also experiencing delays in clinical trials due to shortages of specialized reagents linked to tariffs. In response, the industry is shifting API production to regions like India and Europe, building up inventory reserves, and advocating for tariff exemptions on essential medicines.
The lyophilized injectable drugs market research report is one of a series of new reports that provides lyophilized injectable drugs market statistics, including lyophilized injectable drugs industry global market size, regional shares, competitors with a lyophilized injectable drugs market share, detailed lyophilized injectable drugs market segments, market trends, and opportunities, and any further data you may need to thrive in the lyophilized injectable drugs industry. This lyophilized injectable drugs market research report delivers a complete perspective of everything you need, with an in-depth analysis of the current and future scenario of the industry.
Lyophilized injectable drugs, commonly known as freeze-dried drugs, undergo a process called lyophilization to remove water from the medications. This technique involves freezing the drugs and then subjecting them to a vacuum, causing the frozen water to transition directly from a solid to a gas state. Lyophilized injectable drugs offer improvements in bioavailability, stability, solubility, and patient compliance.
These drugs are packaged in various types, including specialty packaging, point-of-care reconstitution, single-use vials, and others. Point-of-care reconstitution involves preparing medication for immediate use by reconstituting a lyophilized drug at the point of administration or patient bedside. Different delivery methods include single-step devices, multi-step devices, prefilled diluent syringes, and proprietary reconstitution devices. These devices are utilized in the treatment of various conditions such as infectious diseases, autoimmune diseases, and metabolic conditions. The end-users of lyophilized injectable drugs encompass hospitals, ambulatory surgery centers (ASCs), specialty clinics, and other healthcare facilities.
Major companies operating in the lyophilized injectable drugs market include Pfizer Inc., Hoffmann-La Roche Ltd, Merck & Co Inc., Sanofi SA, Novo Nordisk A/S, Becton Dickinson and Company, Baxter International Inc., Sandoz, Nipro Corporation, B. Braun SE, Aurobindo Pharmaceuticals, Schott AG, Zydus Lifesciences, Recipharm AB, Vetter Pharma-Fertigung GmbH & Co. KG, Fresenius Kabi USA LLC, Vetter Pharma-Fertigung GmbH & Co. KG, CordenPharma International, Aristopharma Limited., Accord Healthcare, Jubilant HollisterStier LLC, Ciron Drugs & Pharmaceuticals Pvt Ltd., MSN Laboratories, Qilu pharmaceutical Co Ltd., Genex Pharma.
North America was the largest region in the lyophilized injectable drugs market in 2024. Asia-Pacific is expected to be the fastest-growing region in the forecast period. The regions covered in the lyophilized injectable drugs market report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East, Africa. The countries covered in the lyophilized injectable drugs market report are Australia, Brazil, China, France, Germany, India, Indonesia, Japan, Russia, South Korea, UK, USA, Canada, Italy, Spain.
The lyophilized injectable drugs market consists of revenue by entities by providing Pre-freezing, primary drying, and secondary drying. The market value includes the value of related goods sold by the service provider or included within the service offering. The lyophilized injectable drugs market also includes sales of ceftriaxone, vancomycin, and meropenem. Values in this market are ‘factory gate’ values, that is the value of goods sold by the manufacturers or creators of the goods, whether to other entities (including downstream manufacturers, wholesalers, distributors, and retailers) or directly to end customers. The value of goods in this market includes related services sold by the creators of the goods.
The market value is defined as the revenues that enterprises gain from the sale of goods and/or services within the specified market and geography through sales, grants, or donations in terms of the currency (in USD, unless otherwise specified).
The revenues for a specified geography are consumption values that are revenues generated by organizations in the specified geography within the market, irrespective of where they are produced. It does not include revenues from resales along the supply chain, either further along the supply chain or as part of other products.
This product will be delivered within 1-3 business days.
Table of Contents
Executive Summary
Lyophilized Injectable Drugs Global Market Report 2025 provides strategists, marketers and senior management with the critical information they need to assess the market.This report focuses on lyophilized injectable drugs market which is experiencing strong growth. The report gives a guide to the trends which will be shaping the market over the next ten years and beyond.
Reasons to Purchase:
- Gain a truly global perspective with the most comprehensive report available on this market covering 15 geographies.
- Assess the impact of key macro factors such as geopolitical conflicts, trade policies and tariffs, post-pandemic supply chain realignment, inflation and interest rate fluctuations, and evolving regulatory landscapes.
- Create regional and country strategies on the basis of local data and analysis.
- Identify growth segments for investment.
- Outperform competitors using forecast data and the drivers and trends shaping the market.
- Understand customers based on the latest market shares.
- Benchmark performance against key competitors.
- Suitable for supporting your internal and external presentations with reliable high quality data and analysis
- Report will be updated with the latest data and delivered to you along with an Excel data sheet for easy data extraction and analysis.
- All data from the report will also be delivered in an excel dashboard format.
Description
Where is the largest and fastest growing market for lyophilized injectable drugs? How does the market relate to the overall economy, demography and other similar markets? What forces will shape the market going forward, including technological disruption, regulatory shifts, and changing consumer preferences? The lyophilized injectable drugs market global report answers all these questions and many more.The report covers market characteristics, size and growth, segmentation, regional and country breakdowns, competitive landscape, market shares, trends and strategies for this market. It traces the market’s historic and forecast market growth by geography.
- The market characteristics section of the report defines and explains the market.
- The market size section gives the market size ($b) covering both the historic growth of the market, and forecasting its development.
- The forecasts are made after considering the major factors currently impacting the market. These include: the technological advancements such as AI and automation, Russia-Ukraine war, trade tariffs (government-imposed import/export duties), elevated inflation and interest rates.
- Market segmentations break down the market into sub markets.
- The regional and country breakdowns section gives an analysis of the market in each geography and the size of the market by geography and compares their historic and forecast growth.
- The competitive landscape chapter gives a description of the competitive nature of the market, market shares, and a description of the leading companies. Key financial deals which have shaped the market in recent years are identified.
- The trends and strategies section analyses the shape of the market as it emerges from the crisis and suggests how companies can grow as the market recovers.
Scope
Markets Covered:
1) By Packaging Type: Specialty Packaging, Point-Of-Care Reconstitution, Single-Use Vials, Other Packaging Types.2) By Type of Delivery: Single-Step Devices, Multi-Step Devices, Prefilled Diluent Syringes, Proprietary Reconstitution Devices.
3) By Indication: Infectious Diseases, Autoimmune Diseases, Metabolic Conditions, Other Indications.
4) By End-User: Hospitals, Ambulatory Surgery Centers (ASCs), Specialty Clinics, Other End-Users.
Subsegments:
1) By Specialty Packaging: Dual-Chamber Systems; Prefilled Syringes2) By Point-Of-Care Reconstitution: Portable Reconstitution Devices; Ready-To-Use Kits
3) By Single-Use Vials: Glass Vials; Plastic Vials
4) By Other Packaging Types: Multi-Dose Vials; Blister Packs; Bulk Packaging Solutions
Companies Mentioned: Pfizer Inc.; Hoffmann-La Roche Ltd; Merck & Co Inc.; Sanofi SA; Novo Nordisk a/S; Becton Dickinson and Company; Baxter International Inc.; Sandoz; Nipro Corporation; B. Braun SE; Aurobindo Pharmaceuticals; Schott AG; Zydus Lifesciences; Recipharm AB; Vetter Pharma-Fertigung GmbH & Co. KG; Fresenius Kabi USA LLC; Vetter Pharma-Fertigung GmbH & Co. KG; CordenPharma International; Aristopharma Limited.; Accord Healthcare; Jubilant HollisterStier LLC; Ciron Drugs & Pharmaceuticals Pvt Ltd.; MSN Laboratories; Qilu pharmaceutical Co Ltd.; Genex Pharma
Countries: Australia; Brazil; China; France; Germany; India; Indonesia; Japan; Russia; South Korea; UK; USA; Canada; Italy; Spain
Regions: Asia-Pacific; Western Europe; Eastern Europe; North America; South America; Middle East; Africa
Time Series: Five years historic and ten years forecast.
Data: Ratios of market size and growth to related markets, GDP proportions, expenditure per capita.
Data Segmentation: Country and regional historic and forecast data, market share of competitors, market segments.
Sourcing and Referencing: Data and analysis throughout the report is sourced using end notes.
Delivery Format: PDF, Word and Excel Data Dashboard.
Companies Mentioned
The companies featured in this Lyophilized Injectable Drugs market report include:- Pfizer Inc.
- Hoffmann-La Roche Ltd
- Merck & Co Inc.
- Sanofi SA
- Novo Nordisk A/S
- Becton Dickinson and Company
- Baxter International Inc.
- Sandoz
- Nipro Corporation
- B. Braun SE
- Aurobindo Pharmaceuticals
- Schott AG
- Zydus Lifesciences
- Recipharm AB
- Vetter Pharma-Fertigung GmbH & Co. KG
- Fresenius Kabi USA LLC
- Vetter Pharma-Fertigung GmbH & Co. KG
- CordenPharma International
- Aristopharma Limited.
- Accord Healthcare
- Jubilant HollisterStier LLC
- Ciron Drugs & Pharmaceuticals Pvt Ltd.
- MSN Laboratories
- Qilu pharmaceutical Co Ltd.
- Genex Pharma
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 250 |
| Published | September 2025 |
| Forecast Period | 2025 - 2029 |
| Estimated Market Value ( USD | $ 4 Billion |
| Forecasted Market Value ( USD | $ 4.64 Billion |
| Compound Annual Growth Rate | 3.8% |
| Regions Covered | Global |
| No. of Companies Mentioned | 26 |