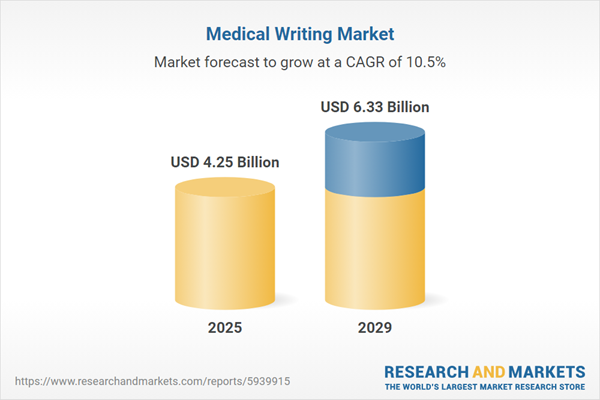
The medical writing market size is expected to see rapid growth in the next few years. It will grow to $6.33 billion in 2029 at a compound annual growth rate (CAGR) of 10.5%. The growth in the forecast period can be attributed to digital transformation in healthcare, the growing trend of remote work and virtual collaboration in medical writing, incorporating patient input into medical writing, the emergence of personalized medicine, increased outsourcing of clinical research, patient-centric communication, integration of real-world data in publications. Major trends in the forecast period include technological advancements, increasing use of artificial intelligence and machine learning in medical writing, partnerships between medical writing agencies, technology providers, and pharmaceutical companies, advancements in digital tools and platforms have streamlined medical writing.
The anticipated growth in the medical writing market is expected to be propelled by the increasing development of new drugs. Novel drugs, defined as pharmaceutical substances not previously approved or marketed for therapeutic use, are a focal point in pharmaceutical advancements. Medical writing contributes significantly to the development of new drugs by providing essential strategies and techniques for the creation of documents necessary in the drug development process. This includes the publication of information related to drug research and information technology. For example, a report published by the Food and Drug Administration in January 2023 revealed that the Center for Drug Evaluation and Research (CDER) granted approval to 37 new drugs in 2022, categorized as novel drugs, indicating substances that had not been previously approved or marketed in the United States. Among these, 20 drugs, accounting for 54%, received approval during the same year. Consequently, the rising development of new drugs is a key driver for the growth of the medical writing market.
The surge in the number of clinical trials is expected to contribute to the growth of the medical writing market. Clinical trials, carefully designed and conducted research studies involving human participants to assess the safety, efficacy, and potential side effects of medical interventions, are on the rise. Medical writing plays a crucial role in all stages of clinical trials, ensuring clear, accurate, and concise communication throughout the research process. This impact extends across various facets of the trial, from planning to publication. As of May 2023, a report by Xtalks indicated that there were 452,604 clinical studies registered on ClinicalTrials.gov, marking a substantial increase from the over 365,000 registered trials recorded in early 2021. Therefore, the upward trend in the number of clinical trials is a significant driver for the growth of the medical writing market.
Product innovation has emerged as a significant trend in the medical writing market, with major companies focusing on developing innovative solutions to strengthen their competitive positions. For instance, in September 2023, Nuance Communications, Inc., a US-based software company, introduced Dragon Ambient eXperience (DAX) Copilot. This AI application utilizes conversational, ambient, and generative AI to automate clinical documentation. DAX Copilot assists physicians in streamlining the documentation process, thereby improving clinical efficiency and reducing burnout by allowing more time for patient care. The tool enables faster and more accurate medical record-keeping, helping healthcare professionals concentrate on delivering high-quality care while enhancing their overall productivity.
Major players in the medical writing market are leveraging advanced technologies, such as AI-based medical writing software, to cater to a broader customer base, drive sales, and increase revenue. An example is Certara, Inc.'s launch of CoAuthor in October 2023. CoAuthor is an AI-based system that provides comprehensive support to medical writers, expediting the development of various clinical trial documents, including protocols, synopses, clinical study reports (CSRs), and patient narratives. The platform facilitates seamless integration of analysis datasets, CDISC data, tables, listings, and figures directly into reports, offering real-time previews and enabling content refresh as needed. CoAuthor establishes a digital data flow, ensuring consistency and saving time through content reuse. Notably, the auto-generated content feature allows writers to choose eTemplates from the library, automatically populating content, headings, styles, and more.
In March 2024, Indegene, an India-based healthcare solutions provider, acquired Trilogy Writing & Consulting for an undisclosed amount. This acquisition is intended to bolster Indegene's capabilities in the medical writing sector and broaden its service offerings to pharmaceutical clients. Trilogy Writing & Consulting, a US-based firm, specializes in medical writing and regulatory consulting, offering expertise in clinical documentation and compliance.
Major companies operating in the medical writing market include Cardinal Health Inc., Merck and Co.Inc., AbbVie Inc., Abbott Laboratories, Medtronic Plc., Labcorp Drug Development Inc., IQVIA Holdings Inc., Icon Plc., Parexel International Corporation, Syneos Health, Ashfield Healthcare Limited, Indegene Pvt. Ltd, UDG Healthcare Plc., Evidera, Certara Inc., MMS Holdings Inc., Freyr Solutions, Cactus Communication Ltd., Omics International Pvt Ltd., Quanticate International limited, Inclin Inc., Criterium Inc., Aptitude Health., Siro Clinpharm Private Limited, ProScribe Medical Communications, Pegasus Knowledge Solutions Inc., Cognibrain, Scientific Communications Group, InkLab Medical Communications, Trilogy Writing and Consulting GmBH.
North America was the largest region in the medical writing market in 2024. Asia-Pacific is expected to be the fastest growing region in the forecast period. The regions covered in the medical writing market report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East, Africa. The countries covered in the medical writing market report are Australia, Brazil, China, France, Germany, India, Indonesia, Japan, Russia, South Korea, UK, USA, Canada, Italy, Spain.
Medical writing is a specialized form of writing that focuses on delivering well-organized and accurate content related to science, medicine, and the healthcare industry. This type of writing produces documents intended to communicate scientific and medical information to specific audiences, including healthcare professionals, regulatory authorities, or the general public.
There are several main types of medical writing, including clinical writing, regulatory writing, scientific writing, and others. Clinical writing is a distinct category within medical writing that encompasses various materials regularly used by healthcare professionals. This includes writing found in research-based documents, scientific papers, journal abstracts, and manuscripts. Clinical writing plays a crucial role in medical journalism, medical education, medico marketing, and other contexts, with pharmaceutical companies, contract research organizations (CROs), and other entities utilizing these written materials.
The medical writing research report is one of a series of new reports that provides medical writing market statistics, including the medical writing industry's global market size, regional shares, competitors with medical writing market share, detailed medical writing market segments, market trends and opportunities, and any further data you may need to thrive in the medical writing industry. This medical writing market research report delivers a complete perspective of everything you need, with an in-depth analysis of the current and future scenarios of the industry.
The medical writing market includes revenues earned by entities by providing services such as consumer health writing, clinical trial reports, social media writing, healthcare marketing materials, patient education materials, scientific posters, and presentations. The market value includes the value of related goods sold by the service provider or included within the service offering. Only goods and services traded between entities or sold to end consumers are included.
The market value is defined as the revenues that enterprises gain from the sale of goods and/or services within the specified market and geography through sales, grants, or donations in terms of the currency (in USD, unless otherwise specified).
The revenues for a specified geography are consumption values that are revenues generated by organizations in the specified geography within the market, irrespective of where they are produced. It does not include revenues from resales along the supply chain, either further along the supply chain or as part of other products.
This product will be delivered within 3-5 business days.
Table of Contents
Executive Summary
Medical Writing Global Market Report 2025 provides strategists, marketers and senior management with the critical information they need to assess the market.This report focuses on medical writing market which is experiencing strong growth. The report gives a guide to the trends which will be shaping the market over the next ten years and beyond.
Reasons to Purchase:
- Gain a truly global perspective with the most comprehensive report available on this market covering 15 geographies.
- Assess the impact of key macro factors such as conflict, pandemic and recovery, inflation and interest rate environment and the 2nd Trump presidency.
- Create regional and country strategies on the basis of local data and analysis.
- Identify growth segments for investment.
- Outperform competitors using forecast data and the drivers and trends shaping the market.
- Understand customers based on the latest market shares.
- Benchmark performance against key competitors.
- Suitable for supporting your internal and external presentations with reliable high quality data and analysis
- Report will be updated with the latest data and delivered to you along with an Excel data sheet for easy data extraction and analysis.
- All data from the report will also be delivered in an excel dashboard format.
Description
Where is the largest and fastest growing market for medical writing? How does the market relate to the overall economy, demography and other similar markets? What forces will shape the market going forward? The medical writing market global report answers all these questions and many more.The report covers market characteristics, size and growth, segmentation, regional and country breakdowns, competitive landscape, market shares, trends and strategies for this market. It traces the market’s historic and forecast market growth by geography.
- The market characteristics section of the report defines and explains the market.
- The market size section gives the market size ($b) covering both the historic growth of the market, and forecasting its development.
- The forecasts are made after considering the major factors currently impacting the market. These include:
- The forecasts are made after considering the major factors currently impacting the market. These include the Russia-Ukraine war, rising inflation, higher interest rates, and the legacy of the COVID-19 pandemic.
- Market segmentations break down the market into sub markets.
- The regional and country breakdowns section gives an analysis of the market in each geography and the size of the market by geography and compares their historic and forecast growth. It covers the growth trajectory of COVID-19 for all regions, key developed countries and major emerging markets.
- The competitive landscape chapter gives a description of the competitive nature of the market, market shares, and a description of the leading companies. Key financial deals which have shaped the market in recent years are identified.
- The trends and strategies section analyses the shape of the market as it emerges from the crisis and suggests how companies can grow as the market recovers.
Scope
Markets Covered:
1) By Type: Clinical Writing; Regulatory Writing; Scientific Writing; Other Types2) By Application: Medical Journalism; Medical Education; Medico Marketing; Other Applications
3) By End-Use: Pharmaceutical Companies; CRO (Contract Research Organization); Other End-Users
Subsegments:
1) By Clinical Writing: Clinical Study Protocols; Clinical Study Reports; Informed Consent Forms2) By Regulatory Writing: Investigational New Drug (IND) Applications; New Drug Applications (NDA); Biologics License Applications (BLA)
3) By Scientific Writing: Manuscripts For Publication; Abstracts and Posters; Literature Reviews
4) By Other Types: Medical Marketing Writing; Educational Materials; Medical Grant Proposals
Key Companies Mentioned: Cardinal Health Inc.; Merck and Co.Inc.; AbbVie Inc.; Abbott Laboratories; Medtronic Plc.
Countries: Australia; Brazil; China; France; Germany; India; Indonesia; Japan; Russia; South Korea; UK; USA; Canada; Italy; Spain
Regions: Asia-Pacific; Western Europe; Eastern Europe; North America; South America; Middle East; Africa
Time Series: Five years historic and ten years forecast.
Data: Ratios of market size and growth to related markets, GDP proportions, expenditure per capita.
Data Segmentation: Country and regional historic and forecast data, market share of competitors, market segments.
Sourcing and Referencing: Data and analysis throughout the report is sourced using end notes.
Delivery Format: PDF, Word and Excel Data Dashboard.
Companies Mentioned
- Cardinal Health Inc.
- Merck and Co.Inc.
- AbbVie Inc.
- Abbott Laboratories
- Medtronic Plc.
- Labcorp Drug Development Inc.
- IQVIA Holdings Inc.
- Icon Plc.
- Parexel International Corporation
- Syneos Health
- Ashfield Healthcare Limited
- Indegene Pvt. Ltd
- UDG Healthcare Plc.
- Evidera
- Certara Inc.
- MMS Holdings Inc.
- Freyr Solutions
- Cactus Communication Ltd.
- Omics International Pvt Ltd.
- Quanticate International limited
- Inclin Inc.
- Criterium Inc.
- Aptitude Health.
- Siro Clinpharm Private Limited
- ProScribe Medical Communications
- Pegasus Knowledge Solutions Inc.
- Cognibrain
- Scientific Communications Group
- InkLab Medical Communications
- Trilogy Writing and Consulting GmBH
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 200 |
| Published | February 2025 |
| Forecast Period | 2025 - 2029 |
| Estimated Market Value ( USD | $ 4.25 Billion |
| Forecasted Market Value ( USD | $ 6.33 Billion |
| Compound Annual Growth Rate | 10.5% |
| Regions Covered | Global |
| No. of Companies Mentioned | 30 |