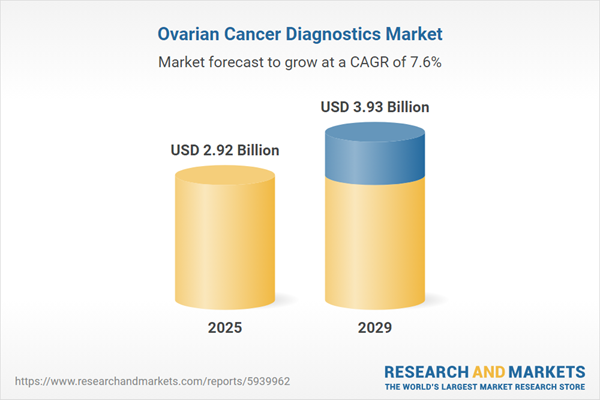
The ovarian cancer diagnostics market size is expected to see strong growth in the next few years. It will grow to $3.93 billion in 2029 at a compound annual growth rate (CAGR) of 7.6%. The growth in the forecast period can be attributed to early detection initiatives, increasing incidence of ovarian cancer, integration of artificial intelligence (AI), personalized medicine approaches, emergence of novel biomarkers, and genomic and proteomic profiling. Major trends in the forecast period include biosensor technologies for early detection, digital pathology integration, innovations in imaging modalities, improved imaging technologies, and advancements in pathology techniques.
The increasing prevalence of ovarian cancer is projected to boost the growth of the ovarian cancer diagnostics market in the future. Ovarian cancer is a malignant tumor that originates in the ovaries, which are the female reproductive organs responsible for producing eggs and hormones. This rise can be attributed to factors such as longer life expectancies, delayed childbearing, and lifestyle changes. Effective ovarian cancer diagnostics play a crucial role in reducing the incidence of the disease by facilitating early detection and intervention, ultimately leading to timely treatment and improved patient outcomes. For example, in 2022, the World Ovarian Cancer, a Canadian organization dedicated to raising awareness for women affected by ovarian cancer globally, reported that by 2050, the global incidence of ovarian cancer among women is anticipated to increase by over 55%, reaching around 503,448 cases. Additionally, the annual mortality rate from ovarian cancer is expected to rise to 350,956, representing an almost 70% increase compared to 2022. Therefore, the growing prevalence of ovarian cancer is driving the expansion of the ovarian cancer diagnostics market.
The rise in gynecological cancer cases is expected to further accelerate the growth of the ovarian cancer diagnostics market. Gynecologic cancer encompasses any cancer that begins in a woman's reproductive organs. Ovarian cancer diagnostics are essential for treating and managing the disease, preventing recurrence, and enhancing patient outcomes through various therapies. For instance, in February 2024, the National Library of Medicine, a US-based biomedical library and national resource for health professionals, reported that excess mortality rates for gynecological cancer increased from 3.30% in 2021 to 8.42% in 2022. Consequently, the high prevalence of gynecological cancer is propelling the growth of the ovarian cancer diagnostics market.
The ovarian cancer diagnostics market is witnessing a notable trend with the increasing adoption of advanced diagnostic technology. Companies in this market are actively incorporating advanced diagnostic technologies as a strategic move to maintain their competitive positions. An illustrative example is Hoffmann-La Roche AG, a pharmaceutical and healthcare company based in Switzerland. In November 2022, the company secured FDA approval for the VENTANA FOLR1 (FOLR1-2.1) RxDx Assay. This groundbreaking immunohistochemistry (IHC)-based companion diagnostic test kit identifies ovarian cancer patients eligible for the prescription medication ELAHERE. The approval signifies a significant advancement in diagnostic capabilities, providing critical insights and detailed clinical information for improved disease management and patient outcomes.
Major players in the ovarian cancer diagnostics market are actively engaging in partnerships to foster product development and fortify their market positions. Collaborative efforts in this sector contribute to enhanced research, development, and commercialization by leveraging the combined expertise, resources, and networks of multiple entities. For instance, in August 2023, ImmunoGen Inc., a US-based biotechnology company, joined forces with Takeda Pharmaceutical Company Limited, a Japan-based pharmaceutical company. This partnership focuses on the development and commercialization of ELAHERE (mirvetuximab soravtansine-gynx) in Japan. ImmunoGen stands to benefit from upfront and milestone payments, along with double-digit royalties, while Takeda gains exclusive rights to develop and commercialize ELAHERE in Japan. This collaboration addresses an unmet need for individuals with platinum-resistant ovarian cancer, showcasing a shared commitment to advancing cancer treatment options on a global scale.
In September 2022, GENinCode PLC, a biotechnology company based in the UK specializing in genetic testing for assessing the risk of cardiovascular diseases, successfully completed the acquisition of Abcodia for a sum of $1.25 million. This strategic acquisition marks a significant expansion, diversification, and reinforcement of GENinCode's technological capabilities. The addition of Abcodia's advanced algorithm technology for the risk assessment of ovarian cancer (ROCA) enhances GENinCode's portfolio, particularly in the domain of early detection of ovarian cancer. Abcodia, also based in the UK, is recognized for its expertise in manufacturing biomarkers designed for the early identification of ovarian cancer. This acquisition underscores GENinCode's commitment to advancing its technology and expanding its offerings, positioning the company as a more comprehensive player in the field of genetic testing and diagnostics.
Major companies operating in the ovarian cancer diagnostics market include F. Hoffmann-La Roche Ltd., Thermo Fisher Scientific Inc., AstraZeneca plc, Abbott Laboratories, GlaxoSmithKline plc, Eli Lilly and Company, GE HealthCare Technologies Inc., Quest Diagnostics Incorporated, Agilent Technologies Inc., Hologic Inc., Illumina Inc., PerkinElmer Inc., Sysmex Corporation, Bio-rad Laboratories Inc., Qiagen N.V., Natera Inc., Myriad Genetics Inc., ArcherDX Inc., Invitae Corporation, Guardant Health, Luminex Corporation, NanoString Technologies Inc., Siemens Healthcare Private Limited, Menarini Silicon Biosystems S.p.A., Precipio Inc., Angle plc.
North America was the largest region in the ovarian cancer diagnostic market in 2024. Asia-Pacific is expected to be the fastest-growing region in the forecast period. The regions covered in the ovarian cancer diagnostics market report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East, Africa. The countries covered in the ovarian cancer diagnostics market report are Australia, Brazil, China, France, Germany, India, Indonesia, Japan, Russia, South Korea, UK, USA, Canada, Italy, Spain.
Ovarian cancer diagnostics encompass a range of methods and procedures aimed at detecting and diagnosing ovarian cancer. This involves the evaluation of symptoms, physical examinations, and the application of various diagnostic tests. Ovarian cancer, a malignancy affecting the ovaries within the female reproductive system, necessitates accurate diagnostic approaches for timely and effective intervention.
The primary categories of ovarian cancer diagnostic products comprise instruments, kits, and reagents. Instruments play a pivotal role in medical diagnostics, encompassing imaging devices such as ultrasound and MRI, blood tests utilizing markers such as CA-125 and HE4, as well as tissue sampling techniques including biopsy and laparoscopy. Various diagnostic methods, such as biopsy, blood tests, imaging, and others, are deployed to identify specific types of ovarian cancer, including epithelial tumors, germ cell tumors, stromal cell tumors, among others. These diagnostic tools find application across diverse end-users, including cancer diagnostic centers, hospital laboratories, and research institutes. The comprehensive array of diagnostic techniques contributes to accurate and targeted identification of ovarian cancer subtypes, facilitating tailored treatment approaches.
The ovarian cancer diagnostics market research report is one of a series of new reports that provides ovarian cancer diagnostics market statistics, including ovarian cancer diagnostics industry global market size, regional shares, competitors with a ovarian cancer diagnostics market share, detailed ovarian cancer diagnostics market segments, market trends and opportunities, and any further data you may need to thrive in the ovarian cancer diagnostics industry. This ovarian cancer diagnostics market research report delivers a complete perspective of everything you need, with an in-depth analysis of the current and future scenario of the industry.
The ovarian cancer diagnostic market includes revenues earned by entities by providing genetic testing, surgery, chemotherapy, target therapy, and hormone therapy. The market value includes the value of related goods sold by the service provider or included within the service offering. The ovarian cancer diagnostic market also includes sales of ultrasound machines, biopsy tools, genetic testing kits, immunohistochemistry (IHC) kits, and ovarian cancer biomarker panels. Values in this market are ‘factory gate’ values, that is the value of goods sold by the manufacturers or creators of the goods, whether to other entities (including downstream manufacturers, wholesalers, distributors and retailers) or directly to end customers. The value of goods in this market includes related services sold by the creators of the goods.
The market value is defined as the revenues that enterprises gain from the sale of goods and/or services within the specified market and geography through sales, grants, or donations in terms of the currency (in USD, unless otherwise specified).
The revenues for a specified geography are consumption values that are revenues generated by organizations in the specified geography within the market, irrespective of where they are produced. It does not include revenues from resales along the supply chain, either further along the supply chain or as part of other products.
This product will be delivered within 3-5 business days.
Table of Contents
Executive Summary
Ovarian Cancer Diagnostics Global Market Report 2025 provides strategists, marketers and senior management with the critical information they need to assess the market.This report focuses on ovarian cancer diagnostics market which is experiencing strong growth. The report gives a guide to the trends which will be shaping the market over the next ten years and beyond.
Reasons to Purchase:
- Gain a truly global perspective with the most comprehensive report available on this market covering 15 geographies.
- Assess the impact of key macro factors such as conflict, pandemic and recovery, inflation and interest rate environment and the 2nd Trump presidency.
- Create regional and country strategies on the basis of local data and analysis.
- Identify growth segments for investment.
- Outperform competitors using forecast data and the drivers and trends shaping the market.
- Understand customers based on the latest market shares.
- Benchmark performance against key competitors.
- Suitable for supporting your internal and external presentations with reliable high quality data and analysis
- Report will be updated with the latest data and delivered to you along with an Excel data sheet for easy data extraction and analysis.
- All data from the report will also be delivered in an excel dashboard format.
Description
Where is the largest and fastest growing market for ovarian cancer diagnostics? How does the market relate to the overall economy, demography and other similar markets? What forces will shape the market going forward? The ovarian cancer diagnostics market global report answers all these questions and many more.The report covers market characteristics, size and growth, segmentation, regional and country breakdowns, competitive landscape, market shares, trends and strategies for this market. It traces the market’s historic and forecast market growth by geography.
- The market characteristics section of the report defines and explains the market.
- The market size section gives the market size ($b) covering both the historic growth of the market, and forecasting its development.
- The forecasts are made after considering the major factors currently impacting the market. These include:
- The forecasts are made after considering the major factors currently impacting the market. These include the Russia-Ukraine war, rising inflation, higher interest rates, and the legacy of the COVID-19 pandemic.
- Market segmentations break down the market into sub markets.
- The regional and country breakdowns section gives an analysis of the market in each geography and the size of the market by geography and compares their historic and forecast growth. It covers the growth trajectory of COVID-19 for all regions, key developed countries and major emerging markets.
- The competitive landscape chapter gives a description of the competitive nature of the market, market shares, and a description of the leading companies. Key financial deals which have shaped the market in recent years are identified.
- The trends and strategies section analyses the shape of the market as it emerges from the crisis and suggests how companies can grow as the market recovers.
Scope
Markets Covered:
1) By Product Type: Instruments; Kits; Reagents2) By Diagnosis Type: Biopsy; Blood Test; Imaging; Other Diagnosis Types
3) By Cancer Type: Epithelial Tumor; Germ Cell Tumor; Stromal Cell Tumor; Other Cancer Types
4) By End User: Cancer Diagnostic Centers; Hospital Laboratories; Research Institutes
Subsegments:
1) By Instruments: Imaging Systems; Biopsy Devices; Molecular Diagnostic Instruments2) By Kits: Diagnostic Test Kits; Screening Kits
3) By Reagents: Antibodies; Enzymes; Assay Reagents
Key Companies Mentioned: F. Hoffmann-La Roche Ltd.; Thermo Fisher Scientific Inc.; AstraZeneca plc; Abbott Laboratories; GlaxoSmithKline plc
Countries: Australia; Brazil; China; France; Germany; India; Indonesia; Japan; Russia; South Korea; UK; USA; Canada; Italy; Spain
Regions: Asia-Pacific; Western Europe; Eastern Europe; North America; South America; Middle East; Africa
Time Series: Five years historic and ten years forecast.
Data: Ratios of market size and growth to related markets, GDP proportions, expenditure per capita.
Data Segmentation: Country and regional historic and forecast data, market share of competitors, market segments.
Sourcing and Referencing: Data and analysis throughout the report is sourced using end notes.
Delivery Format: PDF, Word and Excel Data Dashboard.
Companies Mentioned
- F. Hoffmann-La Roche Ltd.
- Thermo Fisher Scientific Inc.
- AstraZeneca plc
- Abbott Laboratories
- GlaxoSmithKline plc
- Eli Lilly and Company
- GE HealthCare Technologies Inc.
- Quest Diagnostics Incorporated
- Agilent Technologies Inc.
- Hologic Inc.
- Illumina Inc.
- PerkinElmer Inc.
- Sysmex Corporation
- Bio-rad Laboratories Inc.
- Qiagen N.V.
- Natera Inc.
- Myriad Genetics Inc.
- ArcherDX Inc.
- Invitae Corporation
- Guardant Health
- Luminex Corporation
- NanoString Technologies Inc.
- Siemens Healthcare Private Limited
- Menarini Silicon Biosystems S.p.A.
- Precipio Inc.
- Angle plc
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 200 |
| Published | February 2025 |
| Forecast Period | 2025 - 2029 |
| Estimated Market Value ( USD | $ 2.92 Billion |
| Forecasted Market Value ( USD | $ 3.93 Billion |
| Compound Annual Growth Rate | 7.6% |
| Regions Covered | Global |
| No. of Companies Mentioned | 26 |