Global Gastrointestinal OTC Drugs Market Outlook
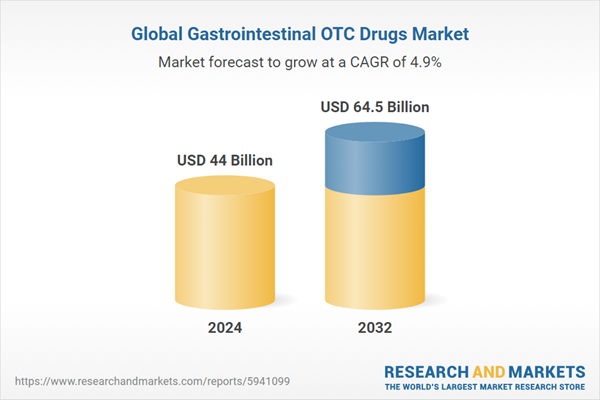
The global gastrointestinal OTC Drugs market size was valued at USD 41.96 billion in 2023, driven by Innovation in drug formulations and delivery systems across the globe. The market size is anticipated to grow at a CAGR of 4.9% during the forecast period of 2024-2032 to achieve a value of USD 64.5 billion by 2032.Gastrointestinal OTC Drugs: Introduction
Gastrointestinal (GI) OTC drugs are medications available without a prescription, used to treat common digestive issues like heartburn, indigestion, constipation, and diarrhea. These include antacids, proton pump inhibitors, laxatives, and anti-diarrheal agents. They offer convenience and quick relief for minor discomforts but are not meant for long-term or serious conditions. It's important to use them as directed and consult a healthcare professional if symptoms persist or worsen, to ensure safe and effective treatment.Key Trends in the Global Gastrointestinal OTC Drugs Market
There's a growing trend of self-medication among consumers for minor GI issues, driven by the accessibility and convenience of OTC drugs. This is supported by the availability of information online, allowing consumers to make informed choices about treating common digestive complaints.Consumers are increasingly seeking natural and organic GI products, perceiving them as safer and with fewer side effects compared to synthetic drugs. This trend is leading to a rise in the availability and popularity of herbal and natural supplements for digestive health.
Innovation in drug formulations and delivery systems, such as chewable tablets, dissolvable strips, and extended-release capsules, is improving the efficacy and convenience of GI OTC drugs. This enhances patient compliance and satisfaction, driving market growth.
There's a growing emphasis on preventive healthcare, leading consumers to use GI OTC drugs not only for treatment but also for preventive measures. Products that contribute to overall digestive health, like probiotics and dietary supplements, are gaining traction.
Regulatory bodies are increasingly scrutinizing OTC drugs to ensure consumer safety. This leads to rigorous labeling requirements and consumer education initiatives about the proper use of OTC drugs and the importance of consulting healthcare professionals for persistent or serious symptoms.
The rise of e-commerce platforms is making GI OTC drugs more accessible, providing consumers with convenient purchasing options. Online pharmacies and health product websites offer a wide range of products, competitive pricing, and the added convenience of home delivery.
Global Gastrointestinal OTC Drugs Market Segmentation
Market Breakup by Class
- Antacids
- Laxatives
- Anti-Diarrheal
- Anti-Emetics
- Others
Market Breakup by Indication
- Heartburn
- Diarrhea
- Constipation
- Gastroesophageal Reflux Disease
- Others
Market Breakup by End User
- Hospitals
- Clinics
- Home Healthcare
- Others
Market Breakup by Region
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East and Africa
Global Gastrointestinal OTC Drugs Market Overview
In North America, the market for gastrointestinal OTC drugs is robust, driven by a culture of self-medication and a high prevalence of digestive disorders related to lifestyle and dietary habits. The U.S. is a significant market, with a wide range of available products and strong consumer trust in OTC solutions for immediate relief. There's also a growing trend towards natural and organic digestive remedies, reflecting the broader shift towards health and wellness in the region.Europe's market is characterized by strong regulatory frameworks ensuring the safety and efficacy of OTC drugs. Consumer preference for OTC solutions for minor gastrointestinal issues is high, and there's a growing demand for products with natural ingredients. The market is also influenced by public health initiatives promoting responsible self-medication and the prudent use of OTC drugs, alongside a focus on preventive healthcare and dietary management to reduce the incidence of digestive issues.
The market in the Asia-Pacific region is expanding rapidly, driven by increasing consumer awareness and improving access to healthcare products. Urbanization, changing lifestyles, and dietary shifts contribute to the prevalence of gastrointestinal issues, fueling the demand for OTC remedies. However, the market is diverse, with variations in consumer preferences and regulatory landscapes. Traditional herbal and natural remedies remain popular in many countries, alongside a growing acceptance of Western OTC gastrointestinal drugs.
Global Gastrointestinal OTC Drugs Market: Competitor Landscape
The key features of the market report include patent analysis, grants analysis, clinical trials analysis, funding and investment analysis, partnerships, and collaborations analysis by the leading key players.- Mylan N.V.
- Sandoz AG
- Johnson & Johnson Services
- Sun Pharmaceuticals Industries
- Teva Pharmaceuticals Industries
- Zydus Cadila
- Sanofi
- Bayer AG
- Pfizer Inc.
- Amneal Pharmaceutical Inc.
- Procter & Gamble
- Hikma Pharmaceutical PLC
- Perrigo Pharmaceutical Plc.
- Abbott
- Lupin
This product will be delivered within 5-7 business days.
Table of Contents
Companies Mentioned
- Mylan N.V.
- Sandoz AG
- Johnson & Johnson Services
- Sun Pharmaceuticals Industries
- Teva Pharmaceuticals Industries
- Zydus Cadila
- Sanofi
- Bayer AG
- Pfizer Inc.
- Amneal Pharmaceutical Inc.
- Procter & Gamble
- Hikma Pharmaceutical PLC
- Perrigo Pharmaceutical Plc.
- Abbott
- Lupin
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 160 |
| Published | February 2024 |
| Forecast Period | 2024 - 2032 |
| Estimated Market Value ( USD | $ 44 Billion |
| Forecasted Market Value ( USD | $ 64.5 Billion |
| Compound Annual Growth Rate | 4.9% |
| Regions Covered | Global |
| No. of Companies Mentioned | 15 |