Diagnostic Laboratory Methods is the fastest growing segment, North America is the largest market globally
Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
Key Market Drivers
The advancements in diagnostic technologies significantly propel the global tuberculosis diagnostics market by enhancing the speed, accuracy, and accessibility of testing solutions. Innovations such as molecular diagnostics, artificial intelligence powered imaging, and next-generation sequencing are transforming the diagnostic landscape, enabling earlier detection and more effective disease management.These technological improvements are particularly crucial for identifying latent tuberculosis infection and for deployment in diverse healthcare settings, from central laboratories to point-of-care environments. For instance, according to a Pharmacy Times news report, in April 2025, the FDA approved the Auto-Pure 2400 liquid handling platform with the T-SPOT. TB test, which allows laboratories to process up to 24 samples in under 3.5 hours for faster and more accurate latent TB diagnosis.
Key Market Challenges
Ensuring affordable and accessible advanced diagnostic technologies in resource-limited regions presents a significant impediment to the expansion of the Global Tuberculosis Diagnostics Market. The high capital expenditure required for advanced molecular diagnostic platforms, coupled with the recurring costs of reagents and consumables, creates substantial financial barriers for public health systems in high-burden, low-income countries. This economic constraint often leads to the underutilization or non-adoption of advanced diagnostic tools despite their accuracy and speed.Key Market Trends
The rise in automated diagnostic platforms significantly drives the global tuberculosis diagnostics market by enhancing testing throughput and operational efficiency. These systems streamline laboratory workflows, minimizing manual intervention and accelerating sample processing, thereby reducing turnaround times for results. By the end of 2023, the Stop TB Partnership reported that cumulative global procurement of GeneXpert machines, representing key automated molecular platforms, exceeded 16,000 units across 167 countries, demonstrating substantial integration into diagnostic infrastructure. This increased capacity is crucial for managing high sample volumes in high-burden settings.Key Market Players Profiled:
- Abbott Laboratories Inc.
- Becton, Dickinson, and Company
- F. Hoffmann-La Roche Ltd.
- Thermo Fisher Scientific Inc.
- BioMérieux SA
- Hain Lifescience GmbH
- QIAGEN NV
- Cepheid
- Hologic, Inc.
Report Scope:
In this report, the Global Tuberculosis Diagnostics Market has been segmented into the following categories:By Test Type:
- Radiographic Method
- Diagnostic Laboratory Methods
- Nucleic Acid Testing
- Phage Assay
- Detection of Latent Infection
- Cytokine Detection Assay
- Detection of Drug Resistance (DST)
- Others
By End-Use:
- Diagnostic Laboratories
- Hospitals & Clinics
- Others
By Region:
- North America
- Europe
- Asia-Pacific
- South America
- MEA
Competitive Landscape
Company Profiles: Detailed analysis of the major companies present in the Global Tuberculosis Diagnostics Market.Available Customizations:
With the given market data, the publisher offers customizations according to a company's specific needs. The following customization options are available for the report:- Detailed analysis and profiling of additional market players (up to five).
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
- Abbott Laboratories Inc.
- Becton, Dickinson, and Company
- F. Hoffmann-La Roche Ltd.
- Thermo Fisher Scientific Inc.
- BioMérieux SA
- Hain Lifescience GmbH
- QIAGEN NV
- Cepheid
- Hologic, Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 182 |
| Published | November 2025 |
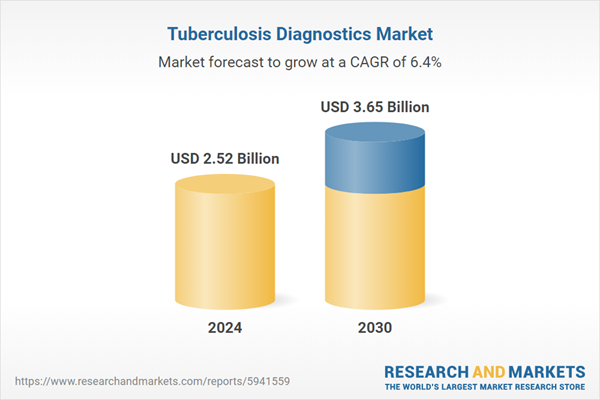
| Forecast Period | 2024 - 2030 |
| Estimated Market Value ( USD | $ 2.52 Billion |
| Forecasted Market Value ( USD | $ 3.65 Billion |
| Compound Annual Growth Rate | 6.3% |
| Regions Covered | Global |
| No. of Companies Mentioned | 9 |