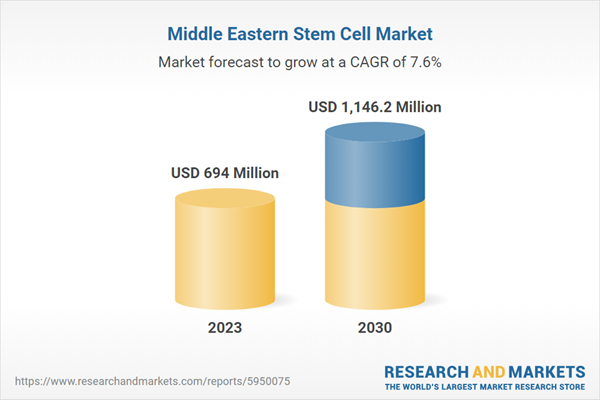
Chapter 1. Methodology and Scope
1.1. Market Segmentation and Scope
1.2. Market Definitions
1.2.1. Product
1.2.2. Application
1.2.3. Technology
1.2.4. Therapy
1.2.5. End-user
1.3. Information analysis
1.4. Market formulation & data visualization
1.5. Data validation & publishing
1.6. Information Procurement
1.6.1. Primary Research
1.7. Information or Data Analysis
1.8. Market Formulation & Validation
1.9. Market Model
1.10. Objectives
Chapter 2. Executive Summary
2.1. Market Outlook
2.2. Segment Snapshot
2.3. Competitive Landscape Snapshot
Chapter 3. Market Variables, Trends, & Scope
3.1. Market Lineage Outlook
3.1.1. Parent Market Outlook
3.1.2. Related/Ancillary Market Outlook
3.2. Market Dynamics
3.2.1. Market Driver Analysis
3.2.1.1. Rising Funds for Accelerating Stem Cell Research
3.2.1.2. Growing Demand for Stem Cell Banking
3.2.1.3. Increasing Prevalence of Genetic Disorders
3.2.2. Market Restraint Analysis
3.2.2.1. Ethical Concern Related to Stem Cell Research
3.2.2.2. Lack of a Well-Defined Regulatory Framework In Certain Applications Of Stem Cell
3.3. Industry Analysis Tools
3.3.1. Porter's Five Forces Analysis
3.3.2. PESTEL Analysis
3.3.3. COVID-19 Impact Analysis
Chapter 4. Middle East Stem Cell Market: Product Business Analysis
4.1. Segment Dashboard
4.2. Middle East Stem Cell Market Product Movement Analysis
4.3. Middle East Stem Cell Market Size & Trend Analysis, by Product, 2018 to 2030 (USD Million)
4.4. Adult Stem Cells (ASCs)
4.4.1. Middle East Adult Stem Cells (ASCs) Market Estimates and Forecasts, 2018 - 2030 (USD Million)
4.4.2. Hematopoietic
4.4.2.1. Middle East Hematopoietic Market Estimates and Forecasts, 2018 - 2030 (USD Million)
4.4.3. Mesenchymal
4.4.3.1. Middle East Mesenchymal Market Estimates and Forecasts, 2018 - 2030 (USD Million)
4.4.4. Neural
4.4.4.1. Middle East Neural Market Estimates and Forecasts, 2018 - 2030 (USD Million)
4.4.5. Epithelial/Skin
4.4.5.1. Middle East Epithelial/Skin Market Estimates and Forecasts, 2018 - 2030 (USD Million)
4.4.6. Others
4.4.6.1. Middle East Others Market Estimates and Forecasts, 2018 - 2030 (USD Million)
4.5. Human Embryonic Stem Cells (HESCs)
4.5.1. Middle East Human Embryonic Stem Cells (HESCs) Market Estimates and Forecasts, 2018 - 2030 (USD Million)
4.6. Induced Pluripotent Stem Cells (iPSCs)
4.6.1. Middle East-Induced Pluripotent Stem Cells (iPSCs) Market Estimates and Forecasts, 2018 - 2030 (USD Million)
4.7. Very Small Embryonic Like Stem Cells
4.7.1. Middle East Very Small Embryonic Like Stem Cells Market Estimates and Forecasts, 2018 - 2030 (USD Million)
Chapter 5. Middle East Stem Cell Market: Application Business Analysis
5.1. Segment Dashboard
5.2. Middle East Stem Cell Market Application Movement Analysis
5.3. Middle East Stem Cell Market Size & Trend Analysis, by Application, 2018 to 2030 (USD Million)
5.4. Regenerative Medicine
5.4.1. Middle East Regenerative Medicine Market Estimates and Forecasts, 2018 - 2030 (USD Million)
5.4.2. Neurology
5.4.2.1. Middle East Neurology Market Estimates and Forecasts, 2018 - 2030 (USD Million)
5.4.3. Orthopedics
5.4.3.1. Middle East Orthopedics Market Estimates and Forecasts, 2018 - 2030 (USD Million)
5.4.4. Oncology
5.4.4.1. Middle East Oncology Market Estimates and Forecasts, 2018 - 2030 (USD Million)
5.4.5. Hematology
5.4.5.1. Middle East Hematology Market Estimates and Forecasts, 2018 - 2030 (USD Million)
5.4.6. Cardiovascular and Myocardial Infraction
5.4.6.1. Middle East Cardiovascular and Myocardial Infraction Market Estimates and Forecasts, 2018 - 2030 (USD Million)
5.4.7. Injuries
5.4.7.1. Middle East Injuries Market Estimates and Forecasts, 2018 - 2030 (USD Million)
5.4.8. Diabetes
5.4.8.1. Middle East Diabetes Market Estimates and Forecasts, 2018 - 2030 (USD Million)
5.4.9. Liver Disorder
5.4.9.1. Middle East Liver Disorder Market Estimates and Forecasts, 2018 - 2030 (USD Million)
5.4.10. Incontinence
5.4.10.1. Middle East Incontinence Market Estimates and Forecasts, 2018 - 2030 (USD Million)
5.4.11. Others
5.4.11.1. Middle East Others Market Estimates and Forecasts, 2018 - 2030 (USD Million)
5.5. Drug Discovery and Development
5.5.1. Middle East Drug Discovery and Development Market Estimates and Forecasts, 2018 - 2030 (USD Million)
Chapter 6. Middle East Stem Cell Market: Technology Business Analysis
6.1. Segment Dashboard
6.2. Middle East Stem Cell Market Technology Movement Analysis
6.3. Middle East Stem Cell Market Size & Trend Analysis, by Technology, 2018 to 2030 (USD Million)
6.4. Cell Acquisition
6.4.1. Middle East Cell Acquisition Market Estimates and Forecasts, 2018 - 2030 (USD Million)
6.4.2. Bone Marrow Harvest
6.4.2.1. Middle East Bone Marrow Harvest Market Estimates and Forecasts, 2018 - 2030 (USD Million)
6.4.3. Umbilical Blood Cord
6.4.3.1. Middle East Umbilical Blood Cord Market Estimates and Forecasts, 2018 - 2030 (USD Million)
6.4.4. Apheresis
6.4.4.1. Middle East Apheresis Market Estimates and Forecasts, 2018 - 2030 (USD Million)
6.5. Cell Production
6.5.1. Middle East Cell Production Market Estimates and Forecasts, 2018 - 2030 (USD Million)
6.5.2. Therapeutic Cloning
6.5.2.1. Middle East Therapeutic Cloning Market Estimates and Forecasts, 2018 - 2030 (USD Million)
6.5.3. In-vitro Fertilization
6.5.3.1. Middle East In-vitro Fertilization Market Estimates and Forecasts, 2018 - 2030 (USD Million)
6.5.4. Cell Culture
6.5.4.1. Middle East Cell Culture Market Estimates and Forecasts, 2018 - 2030 (USD Million)
6.5.5. Isolation
6.5.5.1. Middle East Isolation Market Estimates and Forecasts, 2018 - 2030 (USD Million)
6.6. Cryopreservation
6.6.1. Middle East Cryopreservation Market Estimates and Forecasts, 2018 - 2030 (USD Million)
6.7. Expansion and Sub-Culture
6.7.1. Middle East Expansion and Sub-Culture Market Estimates and Forecasts, 2018 - 2030 (USD Million)
Chapter 7. Middle East Stem Cell Market: Therapy Business Analysis
7.1. Segment Dashboard
7.2. Middle East Stem Cell Market Therapy Movement Analysis
7.3. Middle East Stem Cell Market Size & Trend Analysis, by Therapy, 2018 to 2030 (USD Million)
7.4. Autologous
7.4.1. Middle East Autologous Market Estimates and Forecasts, 2018 - 2030 (USD Million)
7.5. Allogenic
7.5.1. Middle East Allogenic Market Estimates and Forecasts, 2018 - 2030 (USD Million)
Chapter 8. Middle East Stem Cell Market: End-user Business Analysis
8.1. Segment Dashboard
8.2. Middle East Stem Cell Market End-user Movement Analysis
8.3. Middle East Stem Cell Market Size & Trend Analysis, by End-user, 2018 to 2030 (USD Million)
8.4. Pharmaceutical and Biotechnology Companies
8.4.1. Middle East Pharmaceutical and Biotechnology Companies Market Estimates and Forecasts, 2018 - 2030 (USD Million)
8.5. Hospitals & Cell Banks
8.5.1. Middle East Hospitals & Cell Banks Market Estimates and Forecasts, 2018 - 2030 (USD Million)
8.6. Academic & Research Institutes
8.6.1. Middle East Academic & Research Institutes Market Estimates and Forecasts, 2018 - 2030 (USD Million)
Chapter 9. Middle East Stem Cell Market: Country Estimates & Trend Analysis by Product, Application, Technology, Therapy, & End-user
9.1. Country Dashboard
9.2. Market Size & Forecasts and Trend Analysis, 2018 to 2030
9.3. Saudi Arabia
9.3.1. Key Country Dynamics
9.3.2. Competitive Scenario
9.3.3. Regulatory Framework
9.3.4. Saudi Arabia Stem Cell Market, 2018 - 2030 (USD Million)
9.4. UAE
9.4.1. Key Country Dynamics
9.4.2. Competitive Scenario
9.4.3. Regulatory Framework
9.4.4. UAE Stem Cell Market, 2018 - 2030 (USD Million)
9.5. Kuwait
9.5.1. Key Country Dynamics
9.5.2. Competitive Scenario
9.5.3. Regulatory Framework
9.5.4. Kuwait Stem Cell Market, 2018 - 2030 (USD Million)
9.6. Israel
9.6.1. Key Country Dynamics
9.6.2. Competitive Scenario
9.6.3. Regulatory Framework
9.6.4. Israel Stem Cell Market, 2018 - 2030 (USD Million)
Chapter 10. Competitive Landscape
10.1. Participant Categorization
10.2. Strategy Mapping
10.3. Company Market Position Analysis, 2023
10.4. Participant’s Overview
10.4.1. Thermo Fisher Scientific, Inc.
10.4.1.1. Overview
10.4.1.2. Financial Performance (Net Revenue/Sales/EBITDA/Gross Profit)
10.4.1.3. Product Benchmarking
10.4.1.4. Strategic Initiatives
10.4.2. STEMCELL Technologies Inc.
10.4.2.1. Overview
10.4.2.2. Financial Performance (Net Revenue/Sales/EBITDA/Gross Profit)
10.4.2.3. Product Benchmarking
10.4.2.4. Strategic Initiatives
10.4.3. Merck KGaA
10.4.3.1. Overview
10.4.3.2. Financial Performance (Net Revenue/Sales/EBITDA/Gross Profit)
10.4.3.3. Product Benchmarking
10.4.3.4. Strategic Initiatives
10.4.4. CellGenix GmbH
10.4.4.1. Overview
10.4.4.2. Financial Performance (Net Revenue/Sales/EBITDA/Gross Profit)
10.4.4.3. Product Benchmarking
10.4.4.4. Strategic Initiatives
10.4.5. Takara Bio
10.4.5.1. Overview
10.4.5.2. Financial Performance (Net Revenue/Sales/EBITDA/Gross Profit)
10.4.5.3. Product Benchmarking
10.4.5.4. Strategic Initiatives
10.4.6. Lonza
10.4.6.1. Overview
10.4.6.2. Financial Performance (Net Revenue/Sales/EBITDA/Gross Profit)
10.4.6.3. Product Benchmarking
10.4.6.4. Strategic Initiatives
10.4.7. Cellartis AB
10.4.7.1. Overview
10.4.7.2. Financial Performance (Net Revenue/Sales/EBITDA/Gross Profit)
10.4.7.3. Product Benchmarking
10.4.7.4. Strategic Initiatives
10.4.8. ATCC
10.4.8.1. Overview
10.4.8.2. Financial Performance (Net Revenue/Sales/EBITDA/Gross Profit)
10.4.8.3. Product Benchmarking
10.4.8.4. Strategic Initiatives
10.4.9. AcceGen
10.4.9.1. Overview
10.4.9.2. Financial Performance (Net Revenue/Sales/EBITDA/Gross Profit)
10.4.9.3. Product Benchmarking
10.4.9.4. Strategic Initiatives
10.4.10. Cell Applications, Inc.
10.4.10.1. Overview
10.4.10.2. Financial Performance (Net Revenue/Sales/EBITDA/Gross Profit)
10.4.10.3. Product Benchmarking
10.4.10.4. Strategic Initiatives
10.4.11. Bio-Techne
10.4.11.1. Overview
10.4.11.2. Financial Performance (Net Revenue/Sales/EBITDA/Gross Profit)
10.4.11.3. Product Benchmarking
10.4.11.4. Strategic Initiatives
List of Tables
Table 1 List of secondary sources
Table 2 List of abbreviations
Table 3 Middle East stem cell market, by product, 2018 - 2030 (USD Million)
Table 4 Middle East stem cell market, by application, 2018 - 2030 (USD Million)
Table 5 Middle East stem cell market, by technology, 2018 - 2030 (USD Million)
Table 6 Middle East stem cell market, by therapy, 2018 - 2030 (USD Million)
Table 7 Middle East stem cell market, by end-user, 2018 - 2030 (USD Million)
Table 8 Middle East stem cell market, by region, 2018 - 2030 (USD Million)
Table 9 Middle East stem cell market, by country, 2018 - 2030 (USD Million)
Table 10 Middle East stem cell market, by product, 2018 - 2030 (USD Million)
Table 11 Middle East stem cell market, by application, 2018 - 2030 (USD Million)
Table 12 Middle East stem cell market, by technology, 2018 - 2030 (USD Million)
Table 13 Middle East stem cell market, by therapy, 2018 - 2030 (USD Million)
Table 14 Middle East stem cell market, by end-user, 2018 - 2030 (USD Million)
Table 15 Saudi Arabia stem cell market, by product, 2018 - 2030 (USD Million)
Table 16 Saudi Arabia stem cell market, by application, 2018 - 2030 (USD Million)
Table 17 Saudi Arabia stem cell market, by technology, 2018 - 2030 (USD Million)
Table 18 Saudi Arabia stem cell market, by therapy, 2018 - 2030 (USD Million)
Table 19 Saudi Arabia stem cell market, by end-user, 2018 - 2030 (USD Million)
Table 20 UAE stem cell market, by product, 2018 - 2030 (USD Million)
Table 21 UAE stem cell market, by application, 2018 - 2030 (USD Million)
Table 22 UAE stem cell market, by technology, 2018 - 2030 (USD Million)
Table 23 UAE stem cell market, by therapy, 2018 - 2030 (USD Million)
Table 24 UAE stem cell market, by end-user, 2018 - 2030 (USD Million)
Table 25 Kuwait stem cell market, by product, 2018 - 2030 (USD Million)
Table 26 Kuwait stem cell market, by application, 2018 - 2030 (USD Million)
Table 27 Kuwait stem cell market, by technology, 2018 - 2030 (USD Million)
Table 28 Kuwait stem cell market, by therapy, 2018 - 2030 (USD Million)
Table 29 Kuwait stem cell market, by end-user, 2018 - 2030 (USD Million)
Table 30 Israel stem cell market, by product, 2018 - 2030 (USD Million)
Table 31 Israel stem cell market, by application, 2018 - 2030 (USD Million)
Table 32 Israel stem cell market, by technology, 2018 - 2030 (USD Million)
Table 33 Israel stem cell market, by therapy, 2018 - 2030 (USD Million)
Table 34 Israel stem cell market, by end-user, 2018 - 2030 (USD Million)
Table 35 Key companies undergoing expansions
Table 36 Key companies undergoing acquisitions
Table 37 Key companies undergoing collaborations
Table 38 Key companies launching new products/services
Table 39 Key companies undertaking other strategies
List of Figures
Fig. 1 Middle East stem cell market segmentation
Fig. 2 Market research process
Fig. 3 Data triangulation techniques
Fig. 4 Primary research pattern
Fig. 5 Market research approaches
Fig. 6 Value-chain-based sizing & forecasting
Fig. 7 QFD modeling for market share assessment
Fig. 8 Market formulation & validation
Fig. 9 Market outlook
Fig. 10 Segment snapshot
Fig. 11 Competitive landscape snapshot
Fig. 12 Parent market outlook
Fig. 13 Middle East stem cell market driver analysis
Fig. 14 Middle East stem cell market restraint analysis
Fig. 15 Middle East stem cell market: Porter's analysis
Fig. 16 Middle East stem cell market: Product outlook and key takeaways
Fig. 17 Middle East stem cell market: Product market share analysis, 2023 - 2030
Fig. 18 Middle East stem cell by adult stem cells market, 2018 - 2030 (USD Million)
Fig. 19 Middle East stem cell market by hematopoietic market, 2018 - 2030 (USD Million)
Fig. 20 Middle East stem cell by mesenchymal market, 2018 - 2030 (USD Million)
Fig. 21 Middle East stem cell market by neural market, 2018 - 2030 (USD Million)
Fig. 22 Middle East stem cell by epithelial/skin market, 2018 - 2030 (USD Million)
Fig. 23 Middle East stem cell market by others market, 2018 - 2030 (USD Million)
Fig. 24 Middle East stem cell by human embryonic stem cells market, 2018 - 2030 (USD Million)
Fig. 25 Middle East stem cell market by induced pluripotent stem cells market, 2018 - 2030 (USD Million)
Fig. 26 Middle East stem cell by very small embryonic like stem cells market, 2018 - 2030 (USD Million)
Fig. 27 Middle East stem cell market: Application outlook and key takeaways
Fig. 28 Middle East stem cell market: Application market share analysis, 2023 - 2030
Fig. 29 Middle East stem cell by regenerative medicine market, 2018 - 2030 (USD Million)
Fig. 30 Middle East stem cell by neurology market, 2018 - 2030 (USD Million)
Fig. 31 Middle East stem cell by orthopedics market, 2018 - 2030 (USD Million)
Fig. 32 Middle East stem cell by oncology market, 2018 - 2030 (USD Million)
Fig. 33 Middle East stem cell by hematology market, 2018 - 2030 (USD Million)
Fig. 34 Middle East stem cell by cardiovascular and myocardial infraction market, 2018 - 2030 (USD Million)
Fig. 35 Middle East stem cell by injuries market, 2018 - 2030 (USD Million)
Fig. 36 Middle East stem cell by diabetes market, 2018 - 2030 (USD Million)
Fig. 37 Middle East stem cell by liver disorder market, 2018 - 2030 (USD Million)
Fig. 38 Middle East stem cell by incontinence market, 2018 - 2030 (USD Million)
Fig. 39 Middle East stem cell by others market, 2018 - 2030 (USD Million)
Fig. 40 Middle East stem cell by drug discovery and development market, 2018 - 2030 (USD Million)
Fig. 41 Middle East stem cell market: Technology outlook and key takeaways
Fig. 42 Middle East stem cell market: Technology market share analysis, 2023 - 2030
Fig. 43 Middle East stem cell by cell acquisition market, 2018 - 2030 (USD Million)
Fig. 44 Middle East stem cell by bone marrow harvest market, 2018 - 2030 (USD Million)
Fig. 45 Middle East stem cell by umbilical blood cord market, 2018 - 2030 (USD Million)
Fig. 46 Middle East stem cell by apheresis market, 2018 - 2030 (USD Million)
Fig. 47 Middle East stem cell by cell production market, 2018 - 2030 (USD Million)
Fig. 48 Middle East stem cell by therapeutic cloning market, 2018 - 2030 (USD Million)
Fig. 49 Middle East stem cell by In-vitro fertilization market, 2018 - 2030 (USD Million)
Fig. 50 Middle East stem cell by cell culture market, 2018 - 2030 (USD Million)
Fig. 51 Middle East stem cell by isolation market, 2018 - 2030 (USD Million)
Fig. 52 Middle East stem cell by cryopreservation market, 2018 - 2030 (USD Million)
Fig. 53 Middle East stem cell by expansion and sub-culture market, 2018 - 2030 (USD Million)
Fig. 54 Middle East stem cell market: Therapy outlook and key takeaways
Fig. 55 Middle East stem cell market: Therapy market share analysis, 2023 - 2030
Fig. 56 Middle East stem cell by autologous market, 2018 - 2030 (USD Million)
Fig. 57 Middle East stem cell by allogenic market, 2018 - 2030 (USD Million)
Fig. 58 Middle East stem cell market: End-user outlook and key takeaways
Fig. 59 Middle East stem cell market: End-user market share analysis, 2023 - 2030
Fig. 60 Middle East stem cell by pharmaceutical and biotechnology companies market, 2018 - 2030 (USD Million)
Fig. 61 Middle East stem cell by hospitals & cell banks market, 2018 - 2030 (USD Million)
Fig. 62 Middle East stem cell by academic & research institutes market, 2018 - 2030 (USD Million)
Fig. 63 Regional marketplace: Key takeaways
Fig. 64 Regional marketplace: Key takeaways
Fig. 65 Middle East stem cell market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 66 Saudi Arabia key country dynamics
Fig. 67 Saudi Arabia stem cell market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 68 UAE key country dynamics
Fig. 69 UAE stem cell market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 70 Kuwait key country dynamics
Fig. 71 Kuwait stem cell market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 72 Israel key country dynamics
Fig. 73 Israel stem cell market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 74 Key company categorization
Fig. 75 Company market positioning
Fig. 76 Market participant categorization
Fig. 77 Strategy framework