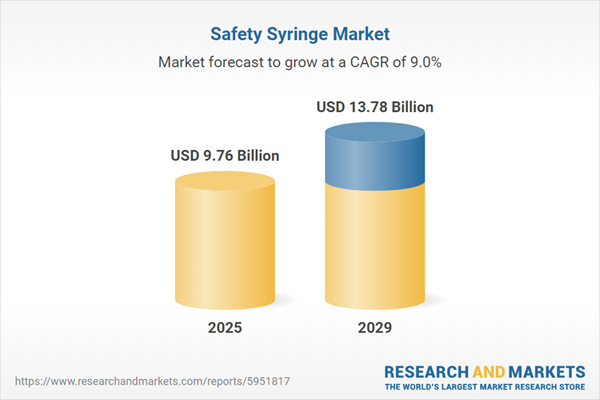
The safety syringe market size has grown strongly in recent years. It will grow from $8.95 billion in 2024 to $9.76 billion in 2025 at a compound annual growth rate (CAGR) of 9.1%. The growth in the historic period can be attributed to needlestick injury concerns, regulatory mandates, increased adoption of safety measures, increasing prevalence of blood-borne diseases, growing healthcare infrastructure.
The safety syringe market size is expected to see strong growth in the next few years. It will grow to $13.78 billion in 2029 at a compound annual growth rate (CAGR) of 9%. The growth in the forecast period can be attributed to vaccination initiatives, rising geriatric population, increased focus on preventive healthcare, expanding biopharmaceutical industry, advancements in material sciences. Major trends in the forecast period include rapid technological innovations, emphasis on sustainable solutions, telehealth integration, customized solutions for specialized therapeutics, enhanced emergency response and preparedness.
The increasing prevalence of blood-borne diseases is expected to drive the growth of the safety syringe market in the future. Blood-borne diseases are infectious conditions caused by microorganisms like bacteria, viruses, or parasites transmitted through the blood. Safety syringes serve as a preventive measure to safeguard healthcare workers and patients from exposure to blood-borne pathogens, thereby reducing the chances of accidental infections and supporting best practices in medical procedures. For instance, in April 2024, the World Health Organization, a Switzerland-based intergovernmental agency, reported that approximately 254 million people were living with chronic hepatitis B infection in 2022, with 1.2 million new cases annually. The disease was responsible for an estimated 1.1 million deaths that year, largely due to cirrhosis and hepatocellular carcinoma (primary liver cancer). Additionally, about 50 million people globally are affected by chronic hepatitis C virus infection, with around 1 million new cases each year. In 2022, hepatitis C led to approximately 242,000 deaths, mainly due to cirrhosis and primary liver cancer. Consequently, the rise in blood-borne diseases is fueling the demand for safety syringes.
Leading companies in the safety syringe market are introducing innovative products, such as glass vaccine syringes, to enhance their competitive position. A glass vaccine syringe is a medical device made from glass, used for delivering vaccines to patients, providing a sterile and reusable option for medication administration. For example, in September 2022, Becton, Dickinson, and Company, a US-based medical device manufacturer, launched the BD Effivax Glass Prefillable Syringe. These prefillable syringes are specifically designed to improve the safety and reliability of drug delivery systems, especially for vaccines. Crafted to ensure accurate medication dosing, they address contamination and product integrity concerns during preparation and administration. Advances in design and manufacturing processes help mitigate risks associated with traditional vial formats, significantly enhancing patient safety in vaccine administration.
In July 2022, Sharps Technology Inc., a medical device company headquartered in the United States, completed the acquisition of a syringe manufacturing facility located in Hungary from SafeGard Medical for $2.5 million. This strategic acquisition is geared towards strengthening Sharps Technology's manufacturing capacities, facilitating the company's transition into revenue-generating commercial activities, and addressing the increasing worldwide demand for its patented smart safety syringes. SafeGard Medical, also based in the US, specializes in emergency medical technologies and training, including the production of safety syringes.
Major companies operating in the safety syringe market report are Cardinal Health Inc., Retractable Technologies Inc., Thermo Fisher Scientific Inc., Medtronic plc, Novo Nordisk A/s, Becton, Dickinson and Company, Baxter International Inc., Boston Scientific Corporation, Medline Industries LP, Terumo Corporation, B. Braun Melsungen AG, West Pharmaceutical Services Inc., Fresenius Kabi AG, Nemera, Smiths Medical International Ltd., Nipro Medical Corporation, Henke-Sass Wolf GmbH, Revolutions Medical Inc., Sol-Millennium Inc., UltiMed Inc., Unilife Corporation, AdvaCare Pharma, Axol Bio Corporation, Vita Needle Company, Numedico Technologies Pty Ltd., Duopross Meditech Corporation, DMC Medical Ltd., Guangdong Haiou Medical Apparatus Co. Ltd., Kendall Healthcare Group Ltd.
North America was the largest region in the safety syringes market in 2024. Asia-Pacific is expected to be the fastest-growing region in the forecast period. The regions covered in the safety syringe market report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East, Africa. The countries covered in the safety syringe market report are Australia, Brazil, China, France, Germany, India, Indonesia, Japan, Russia, South Korea, UK, USA, Canada, Italy, Spain.
The safety syringe market consists of sales of retractable syringes, needleless syringes, safety syringe accessories, needle removers and blood collection devices. Values in this market are ‘factory gate’ values, that is the value of goods sold by the manufacturers or creators of the goods, whether to other entities (including downstream manufacturers, wholesalers, distributors and retailers) or directly to end customers. The value of goods in this market includes related services sold by the creators of the goods.
The market value is defined as the revenues that enterprises gain from the sale of goods and/or services within the specified market and geography through sales, grants, or donations in terms of the currency (in USD, unless otherwise specified).
A safety syringe serves as a medical injection device equipped with integrated safety features to mitigate accidental needle stick injuries or reuse. These syringes are specifically engineered to avert needle stick injuries, which may occur when healthcare workers or individuals inadvertently puncture their skin with a used needle.
Safety syringes primarily comprise retractable safety syringes and non-retractable safety syringes. Retractable safety syringes feature a built-in mechanism enabling the needle to retract or withdraw into the syringe barrel post-injection. They find application in therapies such as insulin, glucagon-like peptide-i (GLP-1), tuberculosis, and growth hormones, catering to subcutaneous and intramuscular administration. These syringes are utilized across various healthcare settings including hospitals, ambulatory surgical centers, long-term care facilities, specialty clinics, among others.
The safety syringe market research report is one of a series of new reports that provides safety syringe market statistics, including safety syringe industry global market size, regional shares, competitors with safety syringe market share, detailed safety syringe market segments, market trends, and opportunities, and any further data you may need to thrive in the safety syringe industry. This safety syringe market research report delivers a complete perspective of everything you need, with an in-depth analysis of the current and future scenarios of the industry.
The revenues for a specified geography are consumption values that are revenues generated by organizations in the specified geography within the market, irrespective of where they are produced. It does not include revenues from resales along the supply chain, either further along the supply chain or as part of other products.
This product will be delivered within 3-5 business days.
Table of Contents
Executive Summary
Safety Syringe Global Market Report 2025 provides strategists, marketers and senior management with the critical information they need to assess the market.This report focuses on safety syringe market which is experiencing strong growth. The report gives a guide to the trends which will be shaping the market over the next ten years and beyond.
Reasons to Purchase:
- Gain a truly global perspective with the most comprehensive report available on this market covering 15 geographies.
- Assess the impact of key macro factors such as conflict, pandemic and recovery, inflation and interest rate environment and the 2nd Trump presidency.
- Create regional and country strategies on the basis of local data and analysis.
- Identify growth segments for investment.
- Outperform competitors using forecast data and the drivers and trends shaping the market.
- Understand customers based on the latest market shares.
- Benchmark performance against key competitors.
- Suitable for supporting your internal and external presentations with reliable high quality data and analysis
- Report will be updated with the latest data and delivered to you along with an Excel data sheet for easy data extraction and analysis.
- All data from the report will also be delivered in an excel dashboard format.
Description
Where is the largest and fastest growing market for safety syringe? How does the market relate to the overall economy, demography and other similar markets? What forces will shape the market going forward? The safety syringe market global report answers all these questions and many more.The report covers market characteristics, size and growth, segmentation, regional and country breakdowns, competitive landscape, market shares, trends and strategies for this market. It traces the market’s historic and forecast market growth by geography.
- The market characteristics section of the report defines and explains the market.
- The market size section gives the market size ($b) covering both the historic growth of the market, and forecasting its development.
- The forecasts are made after considering the major factors currently impacting the market. These include:
- The forecasts are made after considering the major factors currently impacting the market. These include the Russia-Ukraine war, rising inflation, higher interest rates, and the legacy of the COVID-19 pandemic.
- Market segmentations break down the market into sub markets.
- The regional and country breakdowns section gives an analysis of the market in each geography and the size of the market by geography and compares their historic and forecast growth. It covers the growth trajectory of COVID-19 for all regions, key developed countries and major emerging markets.
- The competitive landscape chapter gives a description of the competitive nature of the market, market shares, and a description of the leading companies. Key financial deals which have shaped the market in recent years are identified.
- The trends and strategies section analyses the shape of the market as it emerges from the crisis and suggests how companies can grow as the market recovers.
Scope
Markets Covered:
1) By Product: Retractable Safety Syringes; Non Retractable Safety Syringes2) By Therapy: Insulin; Glucagon-like peptide-I (CLP-I); Tuberculosis; Growth Hormones
3) By Application: Subcutaneous; Intramuscular
4) By End-user: Hospitals; Ambulatory Surgical Centers; Long-term Care Facilities; Specialty Clinics; Other End Users
Subsegments:
1) By Retractable Safety Syringes: Automatic Retractable Safety Syringes; Manual Retractable Safety Syringes2) By Non-Retractable Safety Syringes: Fixed Needle Safety Syringes; Shielded Needle Safety Syringes
Key Companies Mentioned: Cardinal Health Inc.; Retractable Technologies Inc.; Thermo Fisher Scientific Inc.; Medtronic plc; Novo Nordisk A/s
Countries: Australia; Brazil; China; France; Germany; India; Indonesia; Japan; Russia; South Korea; UK; USA; Canada; Italy; Spain
Regions: Asia-Pacific; Western Europe; Eastern Europe; North America; South America; Middle East; Africa
Time Series: Five years historic and ten years forecast.
Data: Ratios of market size and growth to related markets, GDP proportions, expenditure per capita.
Data Segmentation: Country and regional historic and forecast data, market share of competitors, market segments.
Sourcing and Referencing: Data and analysis throughout the report is sourced using end notes.
Delivery Format: PDF, Word and Excel Data Dashboard.
Companies Mentioned
Some of the major companies featured in this Safety Syringe market report include:- Cardinal Health Inc.
- Retractable Technologies Inc.
- Thermo Fisher Scientific Inc.
- Medtronic plc
- Novo Nordisk A/s
- Becton, Dickinson and Company
- Baxter International Inc.
- Boston Scientific Corporation
- Medline Industries LP
- Terumo Corporation
- B. Braun Melsungen AG
- West Pharmaceutical Services Inc.
- Fresenius Kabi AG
- Nemera
- Smiths Medical International Ltd.
- Nipro Medical Corporation
- Henke-Sass Wolf GmbH
- Revolutions Medical iNC.
- Sol-Millennium Inc.
- UltiMed Inc.
- Unilife Corporation
- AdvaCare Pharma
- Axol Bio Corporation
- Vita Needle Company
- Numedico Technologies Pty Ltd.
- Duopross Meditech Corporation
- DMC Medical Ltd.
- Guangdong Haiou Medical Apparatus Co. Ltd.
- Kendall Healthcare Group Ltd.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 200 |
| Published | March 2025 |
| Forecast Period | 2025 - 2029 |
| Estimated Market Value ( USD | $ 9.76 Billion |
| Forecasted Market Value ( USD | $ 13.78 Billion |
| Compound Annual Growth Rate | 9.0% |
| Regions Covered | Global |
| No. of Companies Mentioned | 30 |