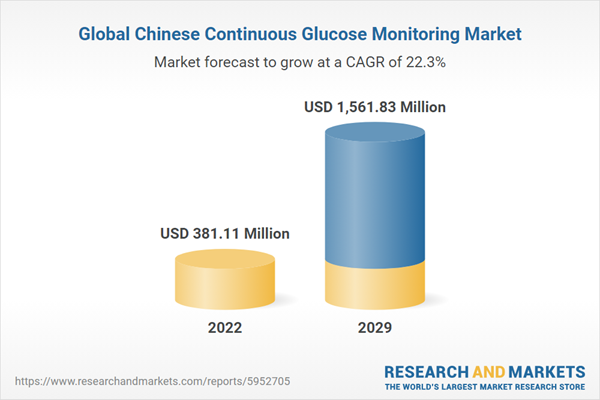
The Chinese continuous glucose monitoring (CGM) market is expected to experience a CAGR of 22.32% throughout the forecast period, reaching a market size of US$1,561.826 million by 2029. This represents a substantial increase from US$381.114 million recorded in 2022.
Continuous glucose monitoring (CGM) is a wearable medical device designed to monitor blood sugar levels continuously, catering to individuals with both type 1 and type 2 diabetes. In China, the CGM market is experiencing significant growth driven by various factors.Key drivers include the escalating prevalence of diabetes, a heightened awareness and acceptance of CGM technology, robust government support, and policy initiatives. Additionally, the increasing disposable income and a growing focus on healthcare contribute to the market's upward trajectory. CGM devices, like other innovative monitoring tools, play a pivotal role in transforming patient care. They empower "remote" patients, allowing them to leverage monitoring devices and wearable sensors, such as continuous glucose monitoring systems (CGMS). These devices not only collect crucial healthcare data but also enable the transmission of this information to relatives and healthcare providers. This facilitates timely interventions, potentially reducing the need for hospital visits. Globally, MicroTech CGMS has proven instrumental in aiding thousands of patients through remote monitoring.
In China, numerous companies, such as SIBIONICS, are making significant strides in the CGM market. SIBIONICS, recognized as the world's third-largest Continuous Glucose Monitoring System (CGMS) brand, has achieved a major milestone by receiving the CE Mark for its revolutionary GS1 CGM. This achievement opens doors for the distribution and adoption of this groundbreaking technology in the European market. The CGM market in China is poised for immense growth, and capitalizing on these driving forces is imperative for sustained expansion. This strategic approach not only ensures market growth but also plays a vital role in enhancing diabetes management for millions of individuals in China.
Rising diabetic cases in China to propel the demand in the market.
The prevalence of Type 2 diabetes in China has experienced a significant surge over the past few decades. In 1980, less than 5% of Chinese men were diagnosed with diabetes. This alarming increase is primarily attributed to unhealthy lifestyles characterized by diets high in sugar and fat, coupled with insufficient physical activity. In 2021, the number of individuals aged 20-79 with diabetes was 140.87 million, a figure projected to escalate to 164.07 million by 2040, as per estimates from the International Diabetes Federation. The gravity of the situation is underscored by the 1.3967 million mortality cases attributable to diabetes in 2021 within the same age group. While increased awareness and improved diagnostics have contributed to higher detection rates, the numbers are expected to rise further due to urbanization, an aging population, and persistently unhealthy lifestyles. Traditionally, diabetes management in China heavily relied on finger-prick glucometers. However, Continuous Glucose Monitoring has emerged as a transformative tool, providing real-time glucose data and trend analysis. This not only facilitates better glycemic control but also enhances the overall quality of life for individuals managing diabetes. The growing recognition of these advantages, coupled with marketing efforts by CGM manufacturers, is propelling the adoption of CGM technology in China. This shift in approach holds promise for improving diabetes management outcomes amidst the rising challenges posed by the escalating rates of Type 2 diabetes in the country.Several factors are influencing the CGM market for diabetic patients in China. The adoption of CGMs is being encouraged by government programs, led by a proactive strategy to combat the growing diabetes pandemic. For instance, in 2019, the Chinese government extended the health system reforms through a range of preventive and capacity reforms, integrating disease prevention and treatment, health management, and health promotion. This required participation from all societal sectors and furthered the promotion of the Healthy China 2030 blueprint. The Diabetes Prevention and Treatment Initiative is one of the 15 main projects included in the Healthy China 2030 blueprint. Innovative solutions are being developed, researched, and put into practice due to cooperative efforts between government agencies, healthcare facilities, and private businesses. The incorporation of CGM technology into all-inclusive diabetes treatment programs has improved through this cooperative approach.
Market Key Developments
- January 2024, a non-binding Letter of Intent was signed between Trinity Biotech plc and Bayer regarding the introduction of a Continuous Glucose Monitoring ("CGM") biosensor device in China and India. This letter of intent relates to the company's recently disclosed purchase of Waveform Technologies, Inc.'s CGM assets.
- November 2023, the third-biggest Continuous Glucose Monitoring System (CGM) brand in China, SIBIONICS, announced that its ground-breaking GS1 CGM has been awarded the CE Mark. With this important milestone, SIBIONICS can distribute and use this revolutionary technology across the European market.
- May 2021, Ascendum Capital, a new healthcare-focused investment platform, Dinova Medtech, a highly specialized medical device innovation hub, and Metronom Health, an inventive U.S. developer of a continuous glucose monitoring (CGM) system, have formed a joint venture in China that specializes in the development, production, and marketing of the CGM system in China. The joint venture's formation will hasten the introduction of Metronom's game-changing CGM technology to China.
Segmentation:
By Application:
- Diabetes Patients
- Critical Patients
By End-User Industry:
- Hospitals
- Diagnostic Centers & Clinics
- Home Care
Table of Contents
Companies Mentioned
- Ypsomed AG
- Dexcom, Inc.
- Abbott Laboratories
- Medtronic plc
- Roche Diabetes Care, Inc.
- Senseonics Holdings, Inc.
- Insulet Corporation
- Tandem Diabetes Care, Inc.
- Zhejiang POCTech Medical Technology Co., Ltd.
- Biolight Meditech Co., Ltd.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 80 |
| Published | February 2024 |
| Forecast Period | 2022 - 2029 |
| Estimated Market Value ( USD | $ 381.11 Million |
| Forecasted Market Value ( USD | $ 1561.83 Million |
| Compound Annual Growth Rate | 22.3% |
| Regions Covered | China |
| No. of Companies Mentioned | 10 |