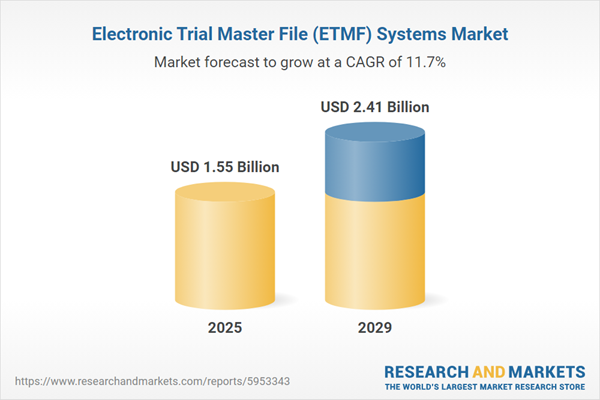
The electronic trial master file (eTMF) systems market size has grown rapidly in recent years. It will grow from $1.36 billion in 2024 to $1.55 billion in 2025 at a compound annual growth rate (CAGR) of 13.9%. The growth in the historic period can be attributed to increasing regulatory compliance requirements, rise in clinical trial complexity, globalization of clinical trials, paper-based document challenges, and increasing emphasis on data quality and integrity.
The electronic trial master file (eTMF) systems market size is expected to see rapid growth in the next few years. It will grow to $2.41 billion in 2029 at a compound annual growth rate (CAGR) of 11.7%. The growth in the forecast period can be attributed to integration with electronic health records (eTMF), rapid growth in clinical trials, focus on patient-centric trials, and emphasis on real-time monitoring and reporting. Major trends in the forecast period include growing demand for AI-powered eTMF systems, increasing focus on data analytics and insights, growing importance of patient-centric trials, and adoption of cloud-based eTMF solutions.
The increase in the number of clinical trials is expected to propel the growth of the electronic trial master file (eTMF) systems market going forward. Clinical trials are systematic investigations or studies conducted to assess the safety, efficacy, and effectiveness of human medical interventions, treatments, or procedures. These trials aim to generate reliable data and evidence to determine whether a new drug, therapy, medical device, or intervention is safe and effective for use in a specific patient population. The upsurge in clinical trials is intricately linked to the widespread adoption of electronic trial master file (eTMF) systems. As the volume and complexity of clinical research expand, eTMF systems play a pivotal role in streamlining documentation processes, ensuring regulatory compliance, and facilitating efficient collaboration among stakeholders. For instance, in November 2023, according to a report published by the Association of the British Pharmaceutical Industry, a UK-based trade association, the total number of clinical trials increased to 411 trials in 2022 and 394 trials in 2021. The annual recruitment to industry clinical trials in the UK is up by 5,366 participants yearly to 42,088 in 2022-23. Therefore, the increase in clinical trials is expected to propel the growth of the electronic trial master file (eTMF) systems market.
Major companies operating in the electronic trial master file (eTMF) systems market are focused on developing innovative products such as cloud-based eTMF to meet the growing demand. Cloud-based eTMF (electronic trial master file) refers to a digital platform or system hosted on cloud infrastructure and specifically designed to manage and organize essential documents and data related to clinical trials in the life sciences and pharmaceutical industries. For instance, in September 2022, Montrium Inc., a Canada-based cloud-based software solutions company, launched cloud-based trial master file (TMF) services and TMF maturity educational training. These offerings are designed to support clinical operations and TMF teams at every stage of the clinical development journey. The comprehensive service offerings are backed by years of experience in TMF processes and cutting-edge technology, leveling the playing field for scaling organizations by allowing them to achieve the highest standard of TMF excellence.
In September 2023, Florence Healthcare Inc., a US-based provider of clinical trial management software solutions, acquired VersaTrial for an undisclosed amount. The acquisition was intended to strengthen site enablement for clinical trials by combining complementary strengths. This strategic initiative aims to optimize the efficiency and capacity of research sites, alleviate the technology load, accelerate feasibility responses, and enhance communication among study personnel. VersaTrial is a clinical trial solutions provider based in the United States.
Major companies operating in the electronic trial master file (eTMF) systems market are Veeva Systems, Oracle, TransPerfect, Phlexglobal, SureClinical Inc., Aurea, Inc., MasterControl, Inc, Clinevo Technologies, Covance Inc., Ennov, Care Lex, ePharma Solutions, Database Integrations, Inc, Aris Global LLC, Mayo Foundation for Medical Education and Research, Montrium Inc., StrelingBio Inc., PHARMAVIGILANT, Forte research, IQVIA, Labcorp Drug Development, NCGD Inc, arivis AG, Wingspan Technology, Dell EMC, Paragon Solutions, Freyr, SAFE-BioPharma, BIOVIA Corp.
North America was the largest region in the electronic trial master file (eTMF) systems market in 2024. Asia-Pacific is expected to be the fastest-growing region in the forecast period. The regions covered in the electronic trial master file (eTMF) systems market report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East, Africa. The countries covered in the electronic trial master file (eTMF) systems market report are Australia, Brazil, China, France, Germany, India, Indonesia, Japan, Russia, South Korea, UK, USA, Canada, Italy, Spain.
The electronic trial master file (eTMF) Systems market includes revenues earned by entities through implementation and integration services, document migration services, quality control and compliance services and regulatory submission support. The market value includes the value of related goods sold by the service provider or included within the service offering. Only goods and services traded between entities or sold to end consumers are included. The electronic trial master file (eTMF) Systems market consists of sales of cloud-based eTMF solutions, eTMF software solutions, mobile applications, collaboration and communication tools. Values in this market are ‘factory gate’ values, that is the value of goods sold by the manufacturers or creators of the goods, whether to other entities (including downstream manufacturers, wholesalers, distributors and retailers) or directly to end customers. The value of goods in this market includes related services sold by the creators of the goods.
The market value is defined as the revenues that enterprises gain from the sale of goods and/or services within the specified market and geography through sales, grants, or donations in terms of the currency (in USD, unless otherwise specified).
An electronic trial master file (eTMF) is a trial master file in electronic or digital format, serving as a content management system for the pharmaceutical industry. The eTMF system manages, stores, tracks, and archives essential clinical study documents electronically. It is designed to ensure compliance with regulatory requirements, such as the FDA's Title 21 CFR Part 11, and to provide a secure, centralized, and easily accessible repository for clinical trial documents.
The main components of electronic trial master file (eTMF) systems are services and services. The electronic trial master file (eTMF) system services refer to functionalities or features provided by the eTMF system to support the management of clinical trial documentation in electronic format. It is delivered on-premise and cloud-based and used in pharmaceutical and biotechnology companies, contract research organizations, and other users.
The main components of electronic trial master file (eTMF) systems are services and services. The electronic trial master file (eTMF) system services refer to functionalities or features provided by the eTMF system to support the management of clinical trial documentation in electronic format. It is delivered on-premise and cloud-based and used in pharmaceutical and biotechnology companies, contract research organizations, and other users.
The revenues for a specified geography are consumption values that are revenues generated by organizations in the specified geography within the market, irrespective of where they are produced. It does not include revenues from resales along the supply chain, either further along the supply chain or as part of other products.
This product will be delivered within 3-5 business days.
Table of Contents
Executive Summary
Electronic Trial Master File (eTMF) Systems Global Market Report 2025 provides strategists, marketers and senior management with the critical information they need to assess the market.This report focuses on electronic trial master file (etmf) systems market which is experiencing strong growth. The report gives a guide to the trends which will be shaping the market over the next ten years and beyond.
Reasons to Purchase:
- Gain a truly global perspective with the most comprehensive report available on this market covering 15 geographies.
- Assess the impact of key macro factors such as conflict, pandemic and recovery, inflation and interest rate environment and the 2nd Trump presidency.
- Create regional and country strategies on the basis of local data and analysis.
- Identify growth segments for investment.
- Outperform competitors using forecast data and the drivers and trends shaping the market.
- Understand customers based on the latest market shares.
- Benchmark performance against key competitors.
- Suitable for supporting your internal and external presentations with reliable high quality data and analysis
- Report will be updated with the latest data and delivered to you along with an Excel data sheet for easy data extraction and analysis.
- All data from the report will also be delivered in an excel dashboard format.
Description
Where is the largest and fastest growing market for electronic trial master file (etmf) systems? How does the market relate to the overall economy, demography and other similar markets? What forces will shape the market going forward? The electronic trial master file (etmf) systems market global report answers all these questions and many more.The report covers market characteristics, size and growth, segmentation, regional and country breakdowns, competitive landscape, market shares, trends and strategies for this market. It traces the market’s historic and forecast market growth by geography.
- The market characteristics section of the report defines and explains the market.
- The market size section gives the market size ($b) covering both the historic growth of the market, and forecasting its development.
- The forecasts are made after considering the major factors currently impacting the market. These include:
- The forecasts are made after considering the major factors currently impacting the market. These include the Russia-Ukraine war, rising inflation, higher interest rates, and the legacy of the COVID-19 pandemic.
- Market segmentations break down the market into sub markets.
- The regional and country breakdowns section gives an analysis of the market in each geography and the size of the market by geography and compares their historic and forecast growth. It covers the growth trajectory of COVID-19 for all regions, key developed countries and major emerging markets.
- The competitive landscape chapter gives a description of the competitive nature of the market, market shares, and a description of the leading companies. Key financial deals which have shaped the market in recent years are identified.
- The trends and strategies section analyses the shape of the market as it emerges from the crisis and suggests how companies can grow as the market recovers.
Scope
Markets Covered:
1) By Component: Services; Software2) By Delivery Mode: On-Premise; Cloud-Based
3) By End-User: Pharmaceutical And Biotechnology Companies; Contract Research Organizations (CROs); Other End-Users
Subsegments:
1) By Services: Consulting Services; Implementation Services; Training And Support Services; Data Migration Services2) By Software: eTMF Management Software; Document Management Software; Cloud-Based eTMF Solutions; Compliance And Regulatory Software
Key Companies Mentioned: Veeva Systems; Oracle; TransPerfect; Phlexglobal; SureClinical Inc.
Countries: Australia; Brazil; China; France; Germany; India; Indonesia; Japan; Russia; South Korea; UK; USA; Canada; Italy; Spain
Regions: Asia-Pacific; Western Europe; Eastern Europe; North America; South America; Middle East; Africa
Time Series: Five years historic and ten years forecast.
Data: Ratios of market size and growth to related markets, GDP proportions, expenditure per capita.
Data Segmentation: Country and regional historic and forecast data, market share of competitors, market segments.
Sourcing and Referencing: Data and analysis throughout the report is sourced using end notes.
Delivery Format: PDF, Word and Excel Data Dashboard.
Companies Mentioned
Some of the major companies featured in this Electronic Trial Master File (ETMF) Systems market report include:- Veeva Systems
- Oracle
- TransPerfect
- Phlexglobal
- SureClinical Inc.
- Aurea, Inc.
- MasterControl, Inc
- Clinevo Technologies
- Covance Inc.
- Ennov
- Care Lex
- ePharma Solutions
- Database Integrations, Inc
- Aris Global LLC
- Mayo Foundation for Medical Education and Research
- Montrium Inc.
- StrelingBio Inc.
- PHARMAVIGILANT
- Forte research
- IQVIA
- Labcorp Drug Development
- NCGD Inc
- arivis AG
- Wingspan Technology
- Dell EMC
- Paragon Solutions
- Freyr
- SAFE-BioPharma
- BIOVIA Corp
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 200 |
| Published | March 2025 |
| Forecast Period | 2025 - 2029 |
| Estimated Market Value ( USD | $ 1.55 Billion |
| Forecasted Market Value ( USD | $ 2.41 Billion |
| Compound Annual Growth Rate | 11.7% |
| Regions Covered | Global |
| No. of Companies Mentioned | 29 |