Genetic Screening of Newborns Fuels the Asia Pacific Newborn Screening Market.
The demand for comprehensive genetic screening of newborns is rising with the deepening knowledge about the genetic causes of medical conditions and advancements in healthcare technologies. As a result, companies in the newborn screening market are developing innovative, cost-effective screening solutions. Yescarta and Zynteglo are two examples of authorized gene treatments for large B-cell lymphoma and beta-thalassemia. Further, the emergence of technologies conferring an ability to identify genetic predispositions to diseases at birth pave the way for personalized healthcare, aligning with the broader trend of precision medicine.
Screening for genetic diseases during pregnancy also focuses on the early detection of pregnancy-related problems. Next-generation sequencing aids in the prenatal screening of neonates with a sensitivity of above 95% for detecting aneuploidies (such as Down syndrome and Trisomy 21) or partial chromosomal abnormalities (duplications or deletions) in all chromosomes. Fluorescence in-situ hybridization (FISH) is employed to detect monogenic illnesses such as sickle cell anemia; it also aids in an effective preimplantation genetic diagnosis. Noninvasive procedures such as the cell-free fetal DNA approach using maternal plasma are the recent advancements in genetic tests. The embryonic DNA can be distinguished from maternal DNA pieces based on differences in their sizes. Real-time PCR with fluorescent probes, shotgun sequencing (Solexa or Illumina), or huge targeted parallel sequencing can be used to examine DNA associated with fetal medical conditions. This would allow doctors to provide early molecular interventions with certain pharmacological therapies (pharmacogenetics) and to transform cells, tissues, and organs physically and chemically if this type of genetic screening is further researched. Thus, advancements in methods for screening infants for genetic disorders hold immense potential for the overall newborn screening market.
Asia Pacific Newborn Screening Market Overview
The newborn screening market in Asia Pacific is segmented into China, Japan, India, South Korea, Australia, and the Rest of Asia Pacific. The Asia -Pacific newborn screening market is segmented into Japan, China, India, South Korea, Australia, and the Rest of Asia -Pacific. Asia -Pacific is expected to be the fastest-growing region in the global newborn screening market during 2022-2030. The projected growth of the market in this region can be associated with the high number of newborn births, the soaring prevalence of congenital diseases, rising awareness regarding early diagnosis of birth defects in newborns, and the presence of organizations working toward offering better solutions for newborn screening.Screening of newborns in Asia -Pacific is gaining traction; government agencies in China, India, and other countries need to implement newborn screening programs owing to the high birth rate. According to the United Nations International Children's Emergency Fund (UNICEF), 67,385 babies are born in India each day, accounting for one-sixth of childbirths recorded globally in a day. In addition, India has become the most populated nation; as per Worldmapper, ~23 million babies were born in 2022, followed by China with the birth of 10 million babies. With the constantly soaring birth rate, the focus on infant care is increasing in India, as well as in other countries in Asia -Pacific.
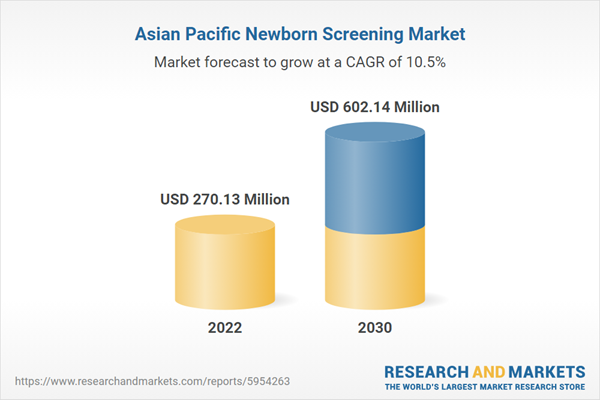
Asi
a Pacific Newborn Screening Market Revenue and Forecast to 2030 (US$ Million)
Asia Pacific Newborn Screening Market Segmentation
The Asia Pacific newborn screening market is segmented based on product type, technology, test type, end user, and country.Based on product type, the Asia Pacific newborn screening market is bifurcated into reagents and assay kits, and instruments. The reagents and assay kits segment held a larger Asia Pacific newborn screening market share in 2022. The reagents and assay kits segment is subsegmented into DNA-based assays, and immunoassays and enzymatic assays. Instruments segment is subsegmented into newborn disorder screening instruments, pulse oximeters, newborn hearing screening instruments, and other instruments.
In terms of technology, the Asia Pacific newborn screening market is categorized into tandem mass spectrometry (TMS), molecular assays, immunoassays and enzymatic assay, pulse oximetry screening technology, and other technologies. The pulse oximetry screening technology segment held the largest Asia Pacific newborn screening market share in 2022.
Based on test type, the Asia Pacific newborn screening market is categorized into dry blood spot test, hearing screen test, critical congenital heart diseases (CCHD) test, and other test types. The dry blood spot test segment held the largest Asia Pacific newborn screening market share in 2022.
By end user, the Asia Pacific newborn screening market is segmented into hospitals and clinics and diagnostic laboratories. The hospitals and clinics segment held a larger Asia Pacific newborn screening market share in 2022.
Based on country, the Asia Pacific newborn screening market is categorized into China, Japan, India, Australia, South Korea, and the Rest of Asia Pacific. China dominated the Asia Pacific newborn screening market in 2022.
Bio-Rad Laboratories Inc, LifeCell International Pvt Ltd, Masimo Corp, Medtronic Plc, Natus Medical Inc, PerkinElmer Inc, Trivitron Healthcare Pvt Ltd, and Waters Corp are some of the leading companies operating in the Asia Pacific newborn screening market.
Reasons to Buy
- Save and reduce time carrying out entry-level research by identifying the growth, size, leading players, and segments in the Asia Pacific newborn screening market.
- Highlights key business priorities in order to assist companies to realign their business strategies
- The key findings and recommendations highlight crucial progressive industry trends in the Asia Pacific newborn screening market, thereby allowing players across the value chain to develop effective long-term strategies
- Develop/modify business expansion plans by using substantial growth offering developed and emerging markets
- Scrutinize in-depth Asia Pacific market trends and outlook coupled with the factors driving the newborn screening market, as well as those hindering it
- Enhance the decision-making process by understanding the strategies that underpin commercial interest with respect to client products, segmentation, pricing, and distribution
Table of Contents
Companies Mentioned
- Bio-Rad Laboratories Inc
- LifeCell International Pvt Ltd
- Masimo Corp
- Medtronic Plc
- Natus Medical Inc
- PerkinElmer Inc
- Trivitron Healthcare Pvt Ltd
- Waters Corp
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 107 |
| Published | February 2024 |
| Forecast Period | 2022 - 2030 |
| Estimated Market Value ( USD | $ 270.13 Million |
| Forecasted Market Value ( USD | $ 602.14 Million |
| Compound Annual Growth Rate | 10.5% |
| Regions Covered | Asia Pacific |
| No. of Companies Mentioned | 8 |