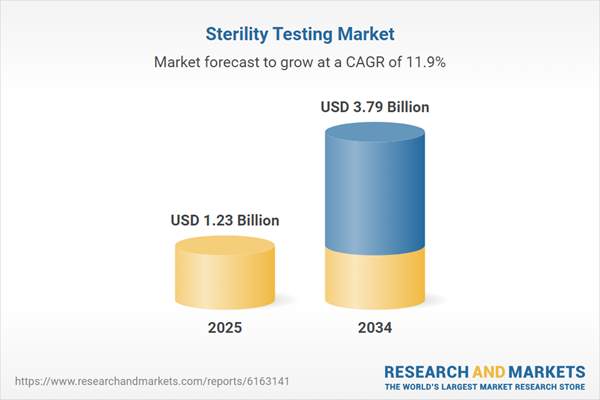
Global Sterility Testing Market Overview
Sterility testing is a mandatory step in the manufacturing process of pharmaceuticals and medical devices. It minimizes the risk of product contamination by verifying the absence of viable microorganisms. Such tests are crucial to ensure the quality, safety, and efficacy of pharmaceutical preparations and medical devices, among others. The healthcare and life science industries have experienced significant growth in production capacity in recent years. This growth is anticipated to contribute to the growing sterility testing market demand in the forecast period.The key players in the market are heavily investing in research and development activities which is positively influencing the market growth. In December 2023, Symbiosis Pharmaceutical Services, a United Kingdom-based contract development and manufacturing organization (CDMO), invested USD 1.25 million to build in-house analytical and microbiological testing capabilities. The new testing laboratories (spanning 3600 square feet) and quality control function are approved by the United Kingdom regulatory body Medicines and Healthcare products Regulatory Agency (MHRA). The project aims to offer sterility, bioburden, and endotoxin testing, among others, to accelerate drug development timelines. Such strategic investment initiatives will significantly aid in the sterility testing market growth.
The rising prevalence and incidence of chronic diseases are also pushing the demand for therapeutics and medical devices that have undergone strict sterility testing. In addition, the rising healthcare expenditure is expected to fuel the market growth.
Global Sterility Testing Market Segmentation
Sterility Testing Market Report and Forecast 2025-2034 offers a detailed analysis of the market based on the following segments:Market Breakup by Products & Services
- Kits And Reagents
- Services
- Instruments
Market Breakup by Test Type
- Membrane Filtration
- Direct Inoculation
Market Breakup by Application
- Pharmaceutical and Biological Manufacturing
- Medical Devices Manufacturing
- Others
Market Breakup by End User
- Pharmaceutical Companies
- Biotechnological Companies
- Others
Market Breakup by Region
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East and Africa
Global Sterility Testing Market: Competitor Landscape
The key features of the market report include patent analysis, grants analysis, funding and investment analysis, partnerships, and collaborations analysis by the leading key players. The major companies in the market are as follows:- Merck KGaA
- BAXTER HEALTHCARE CORPORATION
- BOSTON ANALYTICAL
- LEXAMED LTD
- Danaher Corporation
- Sartorius AG
- Gibraltar Laboratories
- Pace Analytical Services, Inc
- Sigma-Aldrich Co. LLC
- Nelson Laboratories
- Pacific Biolabs.
- BioConvergence LLC
- Thermo Fisher Scientific Inc
- Toxikon Corporation
- Wuxi Apptec
Key Queries Solved in the Global Sterility Testing Market Report
- How will the market landscape evolve in the coming years?
- What are the major market trends influencing the market?
- What are the major drivers, opportunities, and restraints in the market?
- What will be the effect of each driver, challenge, and opportunity on the market?
- Which country is poised to lead the market share in the forecast period?
- Which country is expected to experience expedited growth during the forecast period?
- Which product is anticipated to lead the market share in the forecast period?
- What type of sterility testing services are expected to dominate the respective market segment?
- Which applications are expected to significantly impact the growth of the market?
- What are the key research initiatives expected to boost the sterility testing market value during the forecast period?
- What are the potential risks and challenges associated with the market?
- What are the regulatory requirements governing sterility testing, and how do they influence market dynamics?
- Which segment has the most significant impact on the market size?
- What major mergers and collaborations are poised to impact the market in the forecast period?
This product will be delivered within 3-5 business days.
Table of Contents
Companies Mentioned
- Merck KGaA
- BAXTER HEALTHCARE CORPORATION
- BOSTON ANALYTICAL
- LEXAMED LTD
- Danaher Corporation
- Sartorius AG
- Gibraltar Laboratories
- Pace Analytical Services, Inc
- Sigma-Aldrich Co. LLC
- Nelson Laboratories
- Pacific Biolabs.
- BioConvergence LLC
- Thermo Fisher Scientific Inc
- Toxikon Corporation
- Wuxi Apptec
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 350 |
| Published | July 2025 |
| Forecast Period | 2025 - 2034 |
| Estimated Market Value ( USD | $ 1.23 Billion |
| Forecasted Market Value ( USD | $ 3.79 Billion |
| Compound Annual Growth Rate | 11.9% |
| Regions Covered | Global |
| No. of Companies Mentioned | 15 |