Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
Ongoing innovations in genotyping technologies, including next-generation sequencing (NGS), microarray-based genotyping, polymerase chain reaction (PCR), and capillary electrophoresis, propel market expansion. These advancements facilitate high-throughput, accurate, and cost-effective genotyping assays, broadening their utility across research, diagnostics, and personalized medicine domains. The increasing prevalence of genetic disorders, chronic diseases, and infectious ailments globally underscores the heightened demand for genotyping assays in disease diagnosis, prognosis, and treatment. In the agricultural and animal genomics sectors, genotyping assays play pivotal roles in breeding programs, trait selection, disease resistance profiling, and livestock management. The quest for high-yielding crops, disease-resistant plants, and superior livestock breeds fuels market growth in these domains. As such, genotyping assays emerge as indispensable tools driving advancements across various industries and disciplines.
Key Market Drivers
Advancements in Genotyping Technologies
NGS technologies, also termed high-throughput sequencing, facilitate the rapid sequencing of millions of DNA fragments concurrently. These platforms have substantially decreased the time and cost associated with whole-genome sequencing, exome sequencing, and targeted sequencing, rendering genomic analysis more accessible and cost-effective. Microarray technology enables the simultaneous analysis of numerous genetic variants in a single experiment, commonly employed for GWAS, gene expression profiling, copy number variation analysis, and DNA methylation analysis. Digital PCR offers a highly sensitive and precise method for nucleic acid quantification. Unlike conventional PCR, dPCR segregates a sample into thousands of individual reactions, enabling absolute quantification of target DNA or RNA molecules. This technique is valuable for detecting rare mutations, copy number variations, and gene expression levels with exceptional accuracy. Capillary electrophoresis separates and analyzes DNA fragments based on their size and charge, boasting high resolution, sensitivity, and throughput, making it suitable for genotyping applications like SNP analysis, microsatellite genotyping, and fragment length analysis.Massively Parallel Sequencing (MPS), also termed third-generation sequencing, allows for the simultaneous sequencing of thousands of DNA molecules in real-time. MPS technologies, such as PacBio and Oxford Nanopore sequencing, offer extended read lengths and single molecule sequencing capabilities, ideal for tasks like de novo genome assembly, structural variant detection, and epigenetic analysis. CRISPR-Based Genotyping technology has revolutionized genome editing and genotyping, enabling precise and efficient manipulation of DNA sequences. CRISPR-based genotyping methods enable targeted detection of specific genetic variants and mutations, including allele-specific PCR and high-resolution melting analysis. Multiplex PCR assays simultaneously amplify multiple target DNA sequences in a single reaction, reducing sample input requirements, reaction volumes, and assay time, while increasing throughput and cost-effectiveness. Commonly used for genotyping SNPs, short tandem repeats (STRs), and pathogen detection, multiplex PCR contributes to the development of the Global Genotyping Assay Market.
Increasing Incidence of Genetic Disorders and Chronic Diseases
Genotyping assays play a pivotal role in the early detection and diagnosis of genetic disorders and chronic diseases. By pinpointing specific genetic variants linked to disease susceptibility, progression, and severity, these assays empower healthcare providers to diagnose conditions more accurately and at earlier stages. This enables timely interventions and treatment strategies, enhancing patient outcomes. Genetic insights garnered from genotyping assays facilitate precision medicine approaches, tailoring treatments to individual patients based on their genetic profiles and disease characteristics. Understanding how genetic variations influence drug metabolism, efficacy, and toxicity allows healthcare providers to optimize treatment regimens, improving therapeutic outcomes.Pharmacogenomics, which examines how genes impact an individual's response to drugs, heavily relies on genotyping assays. By scrutinizing genetic variations related to drug metabolism enzymes, targets, and transporters, pharmacogenomic testing aids healthcare providers in predicting medication responses, refining drug selection and dosing, and minimizing the risk of adverse reactions. Genotyping assays assess genetic risk factors linked to various diseases like cardiovascular diseases, cancer, diabetes, and neurodegenerative disorders. Identifying individuals with heightened risk based on their genetic predispositions enables healthcare providers to implement preventive measures, lifestyle adjustments, and personalized screening protocols, curbing disease incidence and enhancing health outcomes.
In family planning and genetic counseling, genotyping assays provide insights into inherited genetic conditions and disease risks. This empowers individuals and families to make informed decisions about family planning, reproductive options, and prenatal screening, effectively managing their genetic health and reducing the transmission of genetic disorders to future generations. The escalating demand for genotyping assays is fueled by the necessity for robust research tools and platforms to explore the genetic underpinnings of diseases, pinpoint novel therapeutic targets, and develop innovative treatment modalities. These assays are vital in pharmacogenomic investigations, biomarker discovery, and clinical trials, facilitating the advancement of personalized therapies and targeted interventions for genetic disorders and chronic diseases. This factor will accelerate the demand for the Global Genotyping Assay Market.
Growing Applications in Agriculture and Animal Genomics
Genotyping assays play a critical role in agriculture, enhancing crop yield, quality, and resilience to various stresses. By analyzing genetic variations linked to desirable traits like disease resistance and drought tolerance, these assays assist plant breeders in identifying superior parental lines, developing new varieties through marker-assisted selection (MAS), and expediting the breeding process. They aid in trait mapping and marker discovery, allowing researchers to understand complex traits' genetic basis and develop molecular markers for marker-assisted breeding (MAB) programs. Furthermore, genotyping assays offer insights into genetic diversity, population structure, and evolutionary history, assisting in preserving genetic resources and designing effective breeding strategies for sustainable agriculture.In animal genomics, genotyping assays evaluate disease resistance, susceptibility, and resilience in livestock populations. By identifying genetic markers associated with disease resistance genes, these assays enable selective breeding programs, the development of disease-resistant animal breeds, and enhanced disease management strategies. They support genomic selection and prediction, aiding breeders in identifying superior individuals, predicting trait performance, and optimizing breeding decisions for faster genetic gains. Genotyping assays also ensure quality control and traceability in agricultural supply chains and food production systems. By verifying the genetic identity and authenticity of seed lots, plant varieties, and livestock breeds, they uphold product integrity, regulatory compliance, and consumer confidence in food safety and quality. This aspect will drive the demand for the Global Genotyping Assay Market.
Key Market Challenges
Data Interpretation and Analysis Complexity
Genotyping assays generate vast amounts of genomic data, including genotypic information for numerous genetic variants across multiple samples. Managing, processing, and analyzing such large data volumes require advanced computational infrastructure and bioinformatics expertise. Interpreting genotyping data involves annotating genetic variants with relevant genomic annotations, functional predictions, and clinical significance. Variant annotation and functional analysis require access to comprehensive databases, annotation tools, and knowledge bases, which may pose challenges in data integration and standardization. Analyzing genotyping data involves performing statistical tests, association analyses, and genotype-phenotype correlations to identify significant genetic associations and candidate variants. Statistical analysis methods may vary depending on study design, population structure, and genetic models, requiring expertise in statistical genetics and bioinformatics. Interpreting genotyping data within the biological context of the studied phenotype or disease requires a deep understanding of molecular genetics, cellular pathways, and biological mechanisms. Integrating genotyping data with functional genomics, transcriptomics, and proteomics datasets enhances biological interpretation but may introduce additional complexity. Communicating genotyping results effectively requires data visualization tools and techniques to present complex genomic data in a clear, interpretable format. Generating informative plots, heatmaps, and interactive visualizations facilitates data exploration, hypothesis generation, and result interpretation for researchers and clinicians.Standardization and Quality Control
Genotyping assays can exhibit variability in experimental conditions, reagents, instrumentation, and data analysis methods, leading to inconsistencies in results across different laboratories and platforms. Standardizing assay protocols and performance metrics is essential to ensure consistency, reliability, and reproducibility of genotyping data. Lack of standardized reference materials and quality control standards poses challenges in assay validation, calibration, and comparison across different genotyping platforms and technologies. Establishing reference materials, certified reference standards, and proficiency testing programs facilitates assay standardization and harmonization, enabling accurate and reliable genotyping results. Maintaining data quality and integrity throughout the genotyping workflow, from sample collection and DNA extraction to data analysis and interpretation, requires robust quality control measures and data validation procedures. Monitoring assay performance, detecting outliers, and implementing data quality checks help identify and mitigate potential sources of error and bias in genotyping data. Inter-laboratory variability in genotyping assays can arise from differences in laboratory practices, equipment calibration, and data analysis pipelines. Collaborative efforts, inter-laboratory comparisons, and proficiency testing programs promote standardization, harmonization, and sharing of best practices among laboratories, enhancing the reliability and comparability of genotyping results. Integrating genotyping data from different platforms, studies, and populations requires harmonization of data formats, quality control metrics, and data annotation standards. Standardized data formats, metadata descriptors, and ontologies facilitate data sharing, meta-analysis, and integration across diverse genotyping datasets, enabling comprehensive genomic research and discovery.Key Market Trends
Increasing Adoption of SNP (Single Nucleotide Polymorphism) Genotyping
SNP genotyping assays enable the simultaneous analysis of hundreds to thousands of SNPs across the genome in a single experiment. High-throughput SNP genotyping platforms, such as microarrays and next-generation sequencing (NGS), facilitate efficient genotyping of large sample cohorts, accelerating genomic research and discovery. SNP genotyping is widely used in genome-wide association studies (GWAS) to identify genetic variants associated with complex traits, diseases, and phenotypic traits. By analyzing SNP frequencies and allele associations across populations, researchers can pinpoint genomic regions linked to disease susceptibility, drug response, and phenotypic variation. SNP genotyping plays a crucial role in precision medicine by enabling the identification of genetic variations that influence individual responses to drugs, treatments, and environmental factors. Pharmacogenomic testing, which analyzes SNP variants in drug metabolism and drug targets, guides personalized treatment decisions, dosage optimization, and medication selection for improved therapeutic outcomes. SNP genotyping allows for the assessment of genetic risk factors associated with common complex diseases, such as cardiovascular diseases, cancer, diabetes, and neurodegenerative disorders. By analyzing disease-associated SNPs and polygenic risk scores, healthcare providers can stratify individuals based on their genetic predispositions, implement preventive measures, and offer personalized health interventions to mitigate disease risk. SNP genotyping is valuable for studying population genetics, evolutionary relationships, and genetic diversity across human populations and species. By analyzing SNP variation within and among populations, researchers can reconstruct population histories, infer migration patterns, and unravel evolutionary processes shaping genetic diversity and adaptation in diverse populations.Segmental Insights
Technology Insights
The Capillary Electrophoresis segment is projected to experience significant growth in the Global Genotyping Assay Market during the forecast period. Capillary electrophoresis (CE) offers high resolution and sensitivity, making it a preferred method for genotyping assays. CE can accurately separate and detect nucleic acids, including DNA fragments and PCR products, based on their size, charge, and mobility, allowing for precise genotyping analysis. Recent advancements in capillary electrophoresis technology have led to the development of automated systems capable of processing large numbers of samples in a high-throughput manner. These automated CE platforms streamline genotyping workflows, improve efficiency, and reduce turnaround times, making them well-suited for applications in research, clinical diagnostics, and drug development. Capillary electrophoresis is a versatile technique that can be adapted to various genotyping assays, including single nucleotide polymorphism (SNP) genotyping, microsatellite analysis, and fragment length analysis. Its flexibility allows researchers and clinicians to customize genotyping assays based on specific research questions, disease markers, or clinical applications. Compared to other genotyping technologies such as next-generation sequencing (NGS) and microarrays, capillary electrophoresis offers a cost-effective solution for medium to high-throughput genotyping applications. The relatively low cost per sample and minimal reagent consumption make CE an attractive option for laboratories with budget constraints or limited resources. Capillary electrophoresis systems are known for their accuracy, reproducibility, and robust performance, ensuring reliable genotyping results across different samples and experimental conditions. The high precision of CE technology makes it suitable for demanding applications in clinical diagnostics, pharmacogenomics, and forensic genotyping.Application Insights
The Pharmacogenomics segment is projected to experience significant growth in the Global Genotyping Assay Market during the forecast period. There is a growing emphasis on personalized medicine, which involves tailoring medical treatments to individual patients based on their genetic makeup, lifestyle, and other factors. Pharmacogenomics, which studies how genes influence an individual's response to drugs, plays a crucial role in personalized medicine by enabling healthcare providers to optimize drug selection, dosage, and treatment regimens based on patients' genetic profiles. Pharmacogenomics helps healthcare providers identify genetic variations that affect drug metabolism, efficacy, and toxicity. By understanding how individual patients are likely to respond to specific medications, healthcare providers can make more informed treatment decisions, leading to improved therapeutic outcomes, reduced adverse drug reactions, and better patient care. Genotyping assays are increasingly being used in drug development and clinical trials to identify genetic biomarkers associated with drug response and treatment outcomes. Pharmaceutical companies utilize pharmacogenomic data to stratify patient populations, identify potential responders and non-responders to investigational drugs, and optimize clinical trial designs, ultimately accelerating the development and approval of new therapeutics. Technological advancements in genotyping technologies, such as next-generation sequencing (NGS), microarray-based genotyping, and digital PCR, have made it easier and more cost-effective to genotype large numbers of genetic variants accurately and efficiently. These advancements have expanded the scope and scalability of pharmacogenomic studies and enabled widespread adoption of genotyping assays in clinical settings.Regional Insights
North America emerged as the dominant player in the Global Genotyping Assay Market in 2023. North America, particularly the United States, is at the forefront of technological advancements in genotyping assays. The region is home to leading biotechnology and pharmaceutical companies, academic research institutions, and innovative startups that drive the development and commercialization of cutting-edge genotyping technologies. North America boasts a robust research and development infrastructure, including state-of-the-art laboratories, research centers, and academic institutions with expertise in genomics, molecular biology, and biotechnology. This ecosystem fosters innovation and collaboration, facilitating the rapid advancement and adoption of genotyping assays. The significant investment in healthcare and life sciences research in North America supports the development and commercialization of genotyping assays. Government funding, private investment, and venture capital funding contribute to the growth of the genotyping assay market by providing resources for research, product development, and market expansion..Report Scope:
In this report, the Global Genotyping Assay Market has been segmented into the following categories, in addition to the industry trends which have also been detailed below:Genotyping Assay Market, By Product & Service:
- Reagents & Kits
- Genotyping Services
- Instruments
- Sequencers & Amplifiers
- Analyzers
- Bioinformatics
- Software
- Services
Genotyping Assay Market, By Technology:
- PCR
- Real-time PCR
- Digital PCR
- Microarrays
- Sequencing
- NGS
- Pyrosequencing
- Sanger Sequencing
- Capillary Electrophoresis
- Amplified Fragment Length Polymorphism
- Restricted Fragment Length Polymorphism
- Single-stranded Conformation Polymorphism
- MALDI-TOF
- Others
Genotyping Assay Market, By Application:
- Pharmacogenomics
- Diagnostic & Personalized Medicine
- Agricultural Biotechnology
- Animal Genetics
- Other Applications
Genotyping Assay Market, By End User:
- Pharmaceutical and Biopharmaceutical Companies
- Diagnostic and Research Laboratories
- Academic Institutes
- Others
Genotyping Assay Market, By Region:
- North America
- United States
- Canada
- Mexico
- Europe
- Germany
- United Kingdom
- France
- Italy
- Spain
- Asia-Pacific
- China
- Japan
- India
- Australia
- South Korea
- South America
- Brazil
- Argentina
- Colombia
- Middle East & Africa
- South Africa
- Saudi Arabia
- UAE
Competitive Landscape
Company Profiles: Detailed analysis of the major companies present in the Global Genotyping Assay Market.Available Customizations:
Global Genotyping Assay market report with the given market data, the publisher offers customizations according to a company's specific needs.This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
- Illumina Inc.
- Thermo Fisher Scientific Inc.
- Qiagen NV
- Agilent Technologies Inc.
- Danaher Corporation
- Hoffmann-La Roche Ltd.
- GE HealthCare Technologies Inc.
- Standard BioTools Inc.
- Eurofins Scientific SE
- Bio-Rad Laboratories Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 182 |
| Published | May 2024 |
| Forecast Period | 2024 - 2029 |
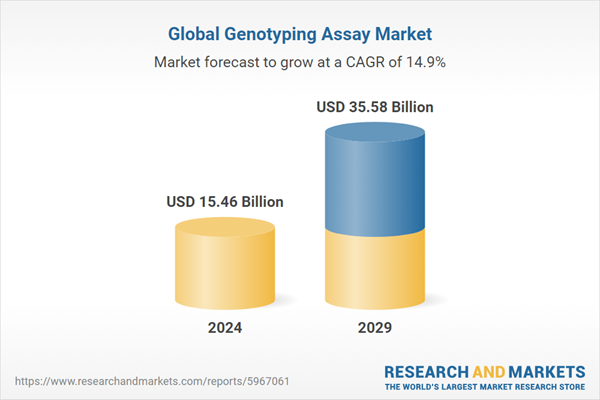
| Estimated Market Value ( USD | $ 15.46 Billion |
| Forecasted Market Value ( USD | $ 35.58 Billion |
| Compound Annual Growth Rate | 14.8% |
| Regions Covered | Global |
| No. of Companies Mentioned | 10 |