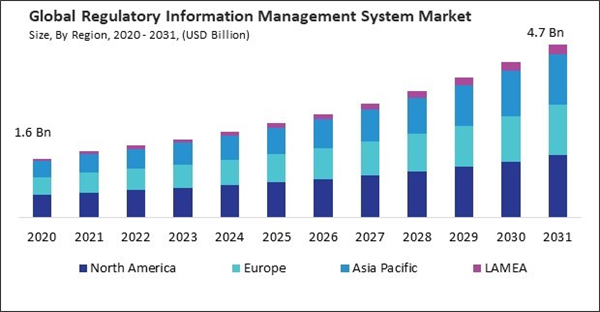
The Asia Pacific region is home to rapidly growing pharmaceutical and healthcare industries. Thus, the Asia Pacific region acquired $579.5 million revenue in 2023. As the pharmaceutical sector expands, the complexity of regulatory compliance requirements also increases, driving the demand for robust regulatory information management systems to ensure compliance with regulatory standards, streamline regulatory processes, and accelerate product approvals in China.
Quality management is paramount in the pharmaceutical and healthcare industries to ensure that products meet safety and efficacy standards. RIMS solutions integrate with Quality Management Systems (QMS) to support quality management processes such as document control, deviation management, corrective and preventive actions (CAPA), and audit management. Therefore, the market is expanding significantly due to the rising focus on patient safety and product quality.
Additionally, RIMS solutions facilitate collaboration among cross-functional teams involved in regulatory processes, including regulatory affairs, quality assurance, clinical research, and product development. RIMS enables teams to collaborate effectively, streamline communication, and coordinate efforts to achieve regulatory objectives by providing a centralized platform for sharing regulatory information, documents, and submissions. Thus, because of the enhanced collaboration and interoperability, the market is anticipated to increase significantly.
However, SMEs often operate with tighter budgets compared to larger corporations. The high upfront costs associated with implementing RIMS solutions may deter SMEs from investing in these systems, limiting their ability to enhance regulatory compliance, streamline processes, and compete effectively in regulated markets. Thus, high implementation costs can slow down the growth of the market.
End User Analysis
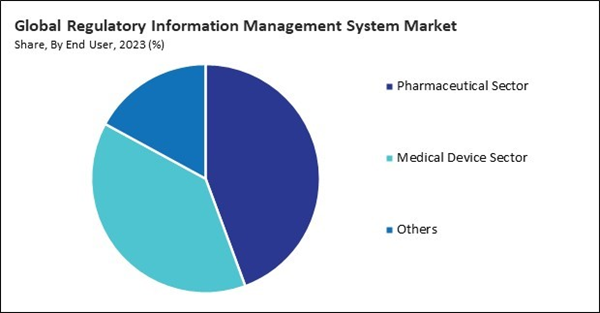
By end user, the market is categorized into pharmaceutical sector, medical device sector, and others. In 2023, the pharmaceutical sector segment held a 44.3% revenue share in the market. Pharmaceutical companies must submit various regulatory documents to health authorities for drug approval and marketing authorization.By Regional Analysis
Region-wise, the market is analysed across North America, Europe, Asia Pacific, and LAMEA. In 2023, the North America region led the market by generating the highest 40% revenue share. Regulatory agencies in North America prioritize patient safety and product quality in approving and overseeing pharmaceuticals, medical devices, and healthcare products.Recent Strategies Deployed in the Market
- 2024-Feb: LORENZ Life Sciences Group came into partnership with DNAnexus, Inc., a company that specializes in cloud-based solutions for genomic data analysis and management. Under this collaboration, LORENZ would be providing Trusted Regulatory Spaces - secure, cloud-native environments enhancing the submission validation process and facilitating sponsor-reviewer communication for human drug and biologics applications to health authorities.
- 2023-Dec: Korber AG signed a partnership with Franz Ziel GmbH, a German engineering company specializing in precision machinery and automation solutions. Through this partnership, Korber would strategically broaden its product range in a key area to better serve our customers.
- 2023-Aug: Veeva Systems, Inc. acquired Learnaboutgmp, an online platform offering comprehensive training and resources for Good Manufacturing Practice (GMP) compliance. Through this acquisition, Veeva would be able to assist clients in fostering more strategic training initiatives, enhancing job competency, and ensuring compliance.
- 2023-May: Veeva Systems, Inc. signed a partnership with Lotus Clinical Research, a leading clinical research organization specializing in conducting trials for pharmaceutical and biotechnology companies. Under this partnership, Veeva would help Lotus to enhance trial efficiency through streamlined collaboration, leveraging user-friendly clinical applications to expand disease expertise and bolster site interactions for enhanced patient recruitment.
- 2023-Apr: Ennov SAS took over Samarind, a company that offers Regulatory Information Management ("RIM") software (known as Samarind RMS) and related services tailored for the life sciences industry. This acquisition would enhance Ennov's standing in the Regulatory Information Management (RIM) software market, particularly within the Medical Device sector.
List of Key Companies Profiled
- Veeva Systems, Inc.
- Korber AG (Optel Group)
- ArisGlobal LLC (Nordic Capital Limited)
- Calyx
- Ennov SAS
- MasterControl, Inc.
- LORENZ Life Sciences Group
- AmpleLogic
- Cencora, Inc. (PharmaLex Holding GmbH)
- Ithos Global Inc. (Cordance Group)
Market Report Segmentation
By End User- Pharmaceutical Sector
- Medical Device Sector
- Others
- North America
- US
- Canada
- Mexico
- Rest of North America
- Europe
- Germany
- UK
- France
- Russia
- Spain
- Italy
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Australia
- Malaysia
- Rest of Asia Pacific
- LAMEA
- Brazil
- Argentina
- UAE
- Saudi Arabia
- South Africa
- Nigeria
- Rest of LAMEA
Table of Contents
Companies Mentioned
- Veeva Systems, Inc.
- Korber AG (Optel Group)
- ArisGlobal LLC (Nordic Capital Limited)
- Calyx
- Ennov SAS
- MasterControl, Inc.
- LORENZ Life Sciences Group
- AmpleLogic
- Cencora, Inc. (PharmaLex Holding GmbH)
- Ithos Global Inc. (Cordance Group)