Healthcare systems in Europe prioritize patient safety, quality of care, and technological innovation. Thus, there is growing recognition of the importance of intraosseous access as a critical tool for rapid vascular access in emergency and critical care settings. Consequently, the Europe region would acquire nearly 30% of the total market share by 2031. Also, Europe boasts a well-developed healthcare infrastructure characterized by advanced medical facilities, robust regulatory frameworks, and high standards of patient care.
In moments of medical crisis, time is of the essence. These devices circumvent delays associated with conventional intravenous access, ensuring healthcare practitioners can promptly administer crucial medications and fluids to stabilize patients. Moreover, the increasing prevalence of medical emergencies underscores the importance of robust emergency response protocols. These devices play a pivotal role in these protocols, providing healthcare teams with a versatile and effective means of vascular access in diverse clinical settings, including pre-hospital environments, emergency departments, and critical care units. Thus, these factors will assist in the growth of the market. Additionally, the utilization of these devices extends beyond traditional emergency settings, permeating critical care units, military operations, and ambulatory care settings. This diversification of usage underscores the versatility of these devices in delivering a spectrum of essential treatments, including medications, fluids, and blood products, across varied healthcare landscapes. Moreover, the rugged and unpredictable nature of military operations necessitates medical interventions that are adaptable and resilient. These devices have emerged as indispensable assets in military healthcare settings, enabling frontline medics to initiate life-saving treatments swiftly and effectively, even in austere environments or under combat conditions. Hence, these aspects will pose lucrative growth prospects for the market.
Moreover, The COVID-19 pandemic placed an unparalleled burden on healthcare systems, resulting in a surge in the need for emergency medical apparatus, such as these devices. Moreover, the pandemic prompted a shift towards pre-hospital and field medicine as healthcare systems adapt to the challenges of managing COVID-19 patients outside traditional hospital settings. These devices became essential tools for emergency medical services (EMS) personnel, military medics, and first responders providing frontline care in community settings, field hospitals, makeshift treatment centers, and mass vaccination sites, where access to advanced medical facilities may be limited. Hence, the COVID-19 pandemic positively impacted the market.
However, the initial investment required for procuring these devices can be substantial, posing a barrier to adoption for healthcare organizations operating under tight budget constraints. Furthermore, beyond initial procurement and training, ongoing maintenance and replacement of consumables constitute recurring expenses associated with these devices. Hence, these aspects can lead to a downturn in the market.
By End User Analysis
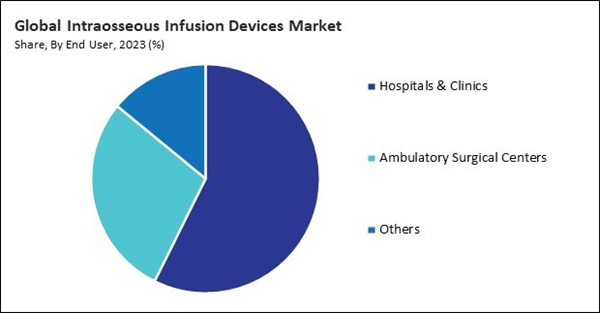
On the basis of end-use, the market is divided into hospitals & clinics, ambulatory surgical centers, and others. In 2023, the ambulatory surgical centers segment witnessed a 28% revenue share in the market. Ambulatory surgical centers have emerged as popular alternatives to traditional hospital-based care for various surgical procedures, including minor surgeries, diagnostic and therapeutic interventions, and emergency procedures. The convenience, efficiency, and cost-effectiveness of ASCs make them attractive options for patients seeking same-day procedures or short-stay surgical care.By Devices Type Analysis
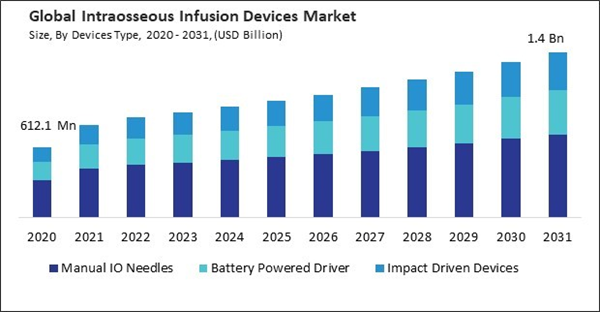
Based on devices type, the market is segmented into manual IO needles, battery powered driver, and impact driven devices. In 2023, the battery powered driver segment garnered a 26% revenue share in the market. Battery-powered drivers represent a significant advancement in intraosseous infusion technology, offering automated and motorized mechanisms for rapid and controlled insertion of intraosseous needles into the bone marrow space. These devices utilize battery-powered motors and mechanisms to drive the needle into the bone with consistent force and precision, minimizing user variability and enhancing procedural efficiency.By Regional Analysis
By region, the market is segmented into North America, Europe, Asia Pacific, and LAMEA. The North America segment procured 40% revenue share in the market in 2023. North America, particularly the United States, faces a high burden of traumatic injuries, emergencies, and acute medical conditions, driving the demand for rapid and reliable vascular access solutions. These devices offer a valuable alternative for obtaining vascular access in critically ill or injured patients when traditional venous access is challenging or unattainable.List of Key Companies Profiled
- Aero Healthcare AU Pty Ltd
- Teleflex Incorporated
- Becton, Dickinson and Company
- BIOPSYBELL S.R.L.
- Cook Medical, Inc.(Cook Group)
- The Seaberg Company Inc. (SAM Medical)
- Argon Medical Devices, Inc. (SHANDONG WEIGAO GROUP MEDICAL POLYMER COMPANY LIMITED)
- Medax Srl
- PAVmed Inc.
Market Report Segmentation
By Devices Type- Manual IO Needles
- Battery Powered Driver
- Impact Driven Devices
- Hospitals & Clinics
- Ambulatory Surgical Centers
- Others
- North America
- US
- Canada
- Mexico
- Rest of North America
- Europe
- Germany
- UK
- France
- Russia
- Spain
- Italy
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Australia
- Malaysia
- Rest of Asia Pacific
- LAMEA
- Brazil
- Argentina
- UAE
- Saudi Arabia
- South Africa
- Nigeria
- Rest of LAMEA
Table of Contents
Companies Mentioned
- Aero Healthcare AU Pty Ltd
- Teleflex Incorporated
- Becton, Dickinson and Company
- BIOPSYBELL S.R.L.
- Cook Medical, Inc.(Cook Group)
- The Seaberg Company Inc. (SAM Medical)
- Argon Medical Devices, Inc. (SHANDONG WEIGAO GROUP MEDICAL POLYMER COMPANY LIMITED)
- Medax Srl
- PAVmed Inc.