The pulmonary embolism market has been comprehensively analyzed in this report titled "Pulmonary Embolism Market: Epidemiology, Industry Trends, Share, Size, Growth, Opportunity, and Forecast 2024-2034". Pulmonary embolism (PE) is a condition that occurs when a blood clot, usually originating in the legs or deep veins of the body, travels to the lungs and blocks one or more of the lung's blood vessels. This can cause damage to the lungs and reduce the amount of oxygen reaching the body's vital organs. PE can be life-threatening if not treated promptly. Symptoms of PE can range from mild to severe, depending on the size and location of the clot. Some of the common symptoms of PE are shortness of breath, chest pain, and coughing up blood. Various other indications may include light-headedness, rapid heartbeat, and leg swelling. Diagnosis of PE typically involves a combination of tests, including a chest X-ray, CT scan, and blood tests. A D-dimer test is also done to see if there is an elevated level of a protein fragment that is produced when a clot is breaking down. Pulmonary embolism treatment typically involves using blood thinners, such as heparin or warfarin, to dissolve the blood clot and prevent further clots from forming. In some cases, catheter-directed thrombolysis or mechanical thrombectomy may be used to remove the clot.
The expanding obese population and the rising incidences of certain inflammatory conditions, such as lupus and rheumatoid arthritis, are primarily driving the global pulmonary embolism market. In addition to this, the growing prevalence of several associated risk factors, such as addiction to smoking, patients undergoing chemotherapy, the inflating usage of supplemental estrogen and hormone replacement therapy, etc., is also bolstering the market growth. Moreover, the widespread adoption of thrombolytic treatment, which utilizes medications to break up existing blood clots, for treating patients with severe lung damage is further creating a positive outlook for the market. Besides this, the emerging popularity of vena cava filters among patients who are unable to take anticoagulant medicines is also augmenting the global market. In this treatment, a small device is inserted into the vena cava (a large vein in the abdomen) to restrict blood clots from traveling to the lungs. Additionally, the elevating utilization of compression stockings for increasing blood flow in the legs in order to prevent the formation of blood clots is acting as another significant growth-inducing factor. Furthermore, the escalating popularity of direct oral anticoagulants (DOACs), which prevent blood clots while having fewer drug-drug interactions and do not require regular monitoring, is also expected to drive the global pulmonary embolism market in the coming years.
This report provides an exhaustive analysis of the pulmonary embolism market in the United States, EU5 (Germany, Spain, Italy, France, and United Kingdom) and Japan. This includes treatment practices, in-market, and pipeline drugs, share of individual therapies, market performance across the seven major markets, market performance of key companies and their drugs, etc. The report also provides the current and future patient pool across the seven major markets. According to the report the United States has the largest patient pool for pulmonary embolism and also represents the largest market for its treatment. Furthermore, the current treatment practice/algorithm, market drivers, challenges, opportunities, reimbursement scenario and unmet medical needs, etc. have also been provided in the report. This report is a must-read for manufacturers, investors, business strategists, researchers, consultants, and all those who have any kind of stake or are planning to foray into the pulmonary embolism market in any manner.
Time Period of the Study
- Base Year: 2023
- Historical Period: 2018-2023
- Market Forecast: 2024-2034
Countries Covered
- United States
- Germany
- France
- United Kingdom
- Italy
- Spain
- Japan
Analysis Covered Across Each Country
- Historical, current, and future epidemiology scenario
- Historical, current, and future performance of the pulmonary embolism market
- Historical, current, and future performance of various therapeutic categories in the market
- Sales of various drugs across the pulmonary embolism market
Competitive Landscape:
This report also provides a detailed analysis of the current pulmonary embolism marketed drugs and late-stage pipeline drugs.In-Market Drugs
- Drug overview
- Mechanism of action
- Regulatory status
- Clinical trial results
- Drug uptake and market performance
Late-Stage Pipeline Drugs
- Drug overview
- Mechanism of action
- Regulatory status
- Clinical trial results
- Drug Uptake and Market Performance
Key Questions Answered in this Report:
Market Insights
- How has the pulmonary embolism market performed so far and how will it perform in the coming years?
- What are the markets shares of various therapeutic segments in 2023 and how are they expected to perform till 2034?
- What was the country-wise size of the pulmonary embolism market across the seven major markets in 2023 and what will it look like in 2034?
- What is the growth rate of the pulmonary embolism market across the seven major markets and what will be the expected growth over the next ten years?
- What are the key unmet needs in the market?
Epidemiology Insights
- What is the number of prevalent cases (2018-2034) of pulmonary embolism across the seven major markets?
- What is the number of prevalent cases (2018-2034) of pulmonary embolism by age across the seven major markets?
- What is the number of prevalent cases (2018-2034) of pulmonary embolism by gender across the seven major markets?
- What is the number of prevalent cases (2018-2034) of pulmonary embolism by type across the seven major markets?
- How many patients are diagnosed (2018-2034) with pulmonary embolism across the seven major markets?
- What is the size of the pulmonary embolism patient pool (2018-2023) across the seven major markets?
- What would be the forecasted patient pool (2024-2034) across the seven major markets?
- What are the key factors driving the epidemiological trend of pulmonary embolism?
- What will be the growth rate of patients across the seven major markets?
Pulmonary Embolism: Current Treatment Scenario, Marketed Drugs and Emerging Therapies
- What are the current marketed drugs and what are their market performance?
- What are the key pipeline drugs and how are they expected to perform in the coming years?
- How safe are the current marketed drugs and what are their efficacies?
- How safe are the late-stage pipeline drugs and what are their efficacies?
- What are the current treatment guidelines for pulmonary embolism drugs across the seven major markets?
- Who are the key companies in the market and what are their market shares?
- What are the key mergers and acquisitions, licensing activities, collaborations, etc. related to the pulmonary embolism market?
- What are the key regulatory events related to the pulmonary embolism market?
- What is the structure of clinical trial landscape by status related to the pulmonary embolism market?
- What is the structure of clinical trial landscape by phase related to the pulmonary embolism market?
- What is the structure of clinical trial landscape by route of administration related to the pulmonary embolism market?
This product will be updated with the latest data at the time of order. Consequently, dispatch time for this product will be 7-10 business days.
Table of Contents
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 136 |
| Published | May 2024 |
| Forecast Period | 2023 - 2034 |
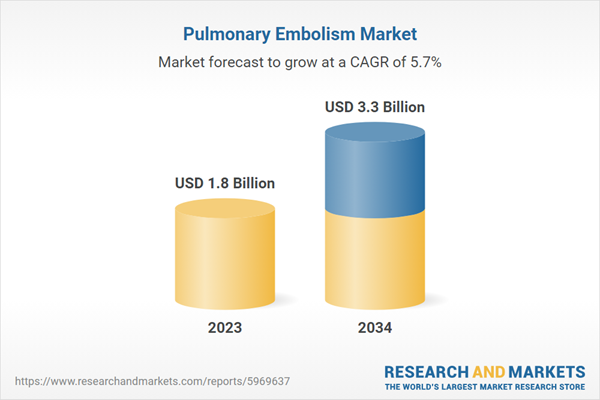
| Estimated Market Value ( USD | $ 1.8 Billion |
| Forecasted Market Value ( USD | $ 3.3 Billion |
| Compound Annual Growth Rate | 5.7% |
| Regions Covered | Global |