The triple-negative breast cancer market has been comprehensively analyzed in this report titled "Triple-negative Breast Cancer Market: Epidemiology, Industry Trends, Share, Size, Growth, Opportunity, and Forecast 2024-2034". Triple-negative breast cancer (TNBC) is a specific type of cancer that tests negative for three major receptors, the hormones estrogen and progesterone, as well as a protein known as human epidermal growth factor receptor 2 (HER2). It is much more malignant than the rest of the cancers and is recognized for being challenging to treat. The symptoms of TNBC are similar to those of other types of breast cancer. The earliest and most typical sign is the formation of a new lump or nodule, which is painless. Other symptoms usually involve swelling in the whole breast, an occurrence of a dimply or saggy outer layer of skin, pain in the breast or the nipple, a discharge in the nipple that might seem to resemble breast milk, reversing nipples, the skin around the nipple or the breast appearing to be dry and brittle, etc. In addition, swollen lymph nodes may often develop in the patient if the breast cancer has migrated to the lymph nodes under the arm or surrounding the collar bone. To diagnose this condition, the clinician will first perform mammography to analyze any appearance of a mass or tumor in the breast. This is followed by a biopsy, during which a tiny part of the breast tissue is extracted and evaluated to determine the subtype of the underlying cancer. The doctor may perform several other tests on the patient, including MRIs, ultrasound scans, computational tomography scans, and positron emission tomography (PET) scans.
The increasing prevalence of breast cancer in women who are under the age of 40 and the growing frequency of reoccurrences of the ailment are primarily driving the triple-negative breast cancer market. In addition to this, the introduction of advanced biopsy techniques that aid in several aspects of breast cancer management, including prognosis prediction, early relapse detection, longitudinal monitoring of disease progress and response to treatment, etc., is further creating a positive outlook for the market. Moreover, the emergence of nanotechnology as an efficient tool in the clinical management of the disease is also bolstering the market growth. The use of nanoparticles facilitates the delivery of drugs and agents to tumor sites in a systematic and efficient manner. Besides this, the ongoing advancements in oncology therapeutics have shifted focus towards an outcome-based approach to cancer care, with a rising emphasis on combination drugs and innovative therapeutic modalities, thereby propelling the global market. Additionally, several key players are making significant investments in developing precise and high-resolution prognostic molecular biomarkers to stratify and distinguish high-risk residual TNBC tumors from low-risk ones. This, in turn, is further acting as another growth-inducing factor. Apart from this, numerous biotechnological developments, including the classification of TNBC into distinct subtypes based on mRNA expression profiles and unique tumor signatures, for generating relevant information about the actionable therapeutic targets, molecular drivers, and effective therapy selection are expected to drive the triple-negative breast cancer market in the coming years.
This report provides an exhaustive analysis of the triple-negative breast cancer market in the United States, EU4 (Germany, Spain, Italy, and France), United Kingdom, and Japan. This includes treatment practices, in-market, and pipeline drugs, share of individual therapies, market performance across the seven major markets, market performance of key companies and their drugs, etc. The report also provides the current and future patient pool across the seven major markets. According to the report, the United States has the largest patient pool for triple-negative breast cancer and also represents the largest market for its treatment. Furthermore, the current treatment practice/algorithm, market drivers, challenges, opportunities, reimbursement scenario, unmet medical needs, etc., have also been provided in the report. This report is a must-read for manufacturers, investors, business strategists, researchers, consultants, and all those who have any kind of stake or are planning to foray into the triple-negative breast cancer market in any manner.
Recent Developments:
In February 2024, a pre-clinical study conducted by the University of Adelaide revealed that CDDD11-8 can successfully inhibit the growth of triple-negative breast cancer without any toxic side effects. This therapeutic candidate is designed to be taken orally, and acts by targeting a specific protein in the cancerous tumor called CDK9, which speeds up cell growth.
In December 2023, Anixa Biosciences, Inc. announced new and updated positive results from the Phase 1 clinical trial of its breast cancer vaccine. The trial is being conducted in collaboration with Cleveland Clinic and supported by a grant from the U.S. Department of Defense. This vaccine is designed to direct the immune system to destroy TNBC cancer cells in patients.
In May 2023, Shanghai Junshi Biosciences Co., Ltd disclosed that the National Medical Products Administration (“NMPA”) had authorized the supplemental new drug application (“sNDA”) for the company's anti-PD-1 monoclonal antibody, toripalimab. This antibody is used in conjunction with albumin-bound paclitaxel to treat PD-L1 positive (CPS ≥ 1) untreated metastatic or recurrent metastatic triple-negative breast cancer.
Key Highlights:
Triple-negative breast cancer represents 10-15 % of all breast cancers and has a low survival rate of less than 5 years.
A maximum of fifty percent of patients diagnosed with early-stage triple-negative breast cancer (stages I to III) develop disease recurrence, and 37% die within the first 5 years of surgery.
It is estimated that 2.6 million new breast cancer cases will occur in 2030, representing an 18 % increase from 2020 globally.
According to studies, Black women are more prone than white women to acquire triple-negative breast cancer, as do women under the age of 40 compared to those aged between 50-64 years.
Around 70%-80% of the breast cancers that develop in women with mutations in the BRCA1 genes are triple-negative breast cancer.
Drugs:
TRODELVY (sacituzumab govitecan) is a prescription medication used to treat women with triple-negative breast cancer (negative for progesterone and estrogen hormone receptors and HER2) that has spread to various other parts of the body (metastatic) or cannot be removed surgically, and who have undergone two or more prior treatments, including at least one for metastatic disease. Sacituzumab govitecan is a Trop-2-directed antibody and topoisomerase inhibitor drug conjugate, which targets the Trop-2 receptor that helps cancer grow, divide, and spread.
Toripalimab is an anti-PD-1 monoclonal antibody used for treating advanced triple-negative breast cancer. It promotes the immune system's ability to attack and kill tumor cells by blocking PD-1 interactions with its ligands, PD-L1 and PD-L2, and for enhanced receptor internalization (endocytosis function).
Mirvetuximab soravtansine is under clinical study by ImmunoGen for triple-negative breast cancer. It is formulated as an intravenous (IV) solution.The drug candidate is an immunoconjugate consisting of the humanized monoclonal antibody that selectively binds to folate receptor 1 (FOLR1).
Time Period of the Study
- Base Year: 2023
- Historical Period: 2018-2023
- Market Forecast: 2024-2034
Countries Covered
- United States
- Germany
- France
- United Kingdom
- Italy
- Spain
- Japan
Analysis Covered Across Each Country
- Historical, current, and future epidemiology scenario
- Historical, current, and future performance of the Triple-negative breast cancer market
- Historical, current, and future performance of various therapeutic categories in the market
- Sales of various drugs across the Triple-negative breast cancer market
Competitive Landscape:
This report also provides a detailed analysis of the current Triple-negative breast cancer marketed drugs and late-stage pipeline drugs.In-Market Drugs
- Drug Overview
- Mechanism of Action
- Regulatory Status
- Clinical Trial Results
- Drug Uptake and Market Performance
Late-Stage Pipeline Drugs
- Drug Overview
- Mechanism of Action
- Regulatory Status
- Clinical Trial Results
- Drug Uptake and Market Performance
Key Questions Answered in this Report:
Market Insights
- How has the Triple-negative breast cancer market performed so far and how will it perform in the coming years?
- What are the markets shares of various therapeutic segments in 2023 and how are they expected to perform till 2034?
- What was the country-wise size of the Triple-negative breast cancer market across the seven major markets in 2023 and what will it look like in 2034?
- What is the growth rate of the Triple-negative breast cancer market across the seven major markets and what will be the expected growth over the next ten years?
- What are the key unmet needs in the market?
Epidemiology Insights
- What is the number of prevalent cases (2018-2034) of Triple-negative breast cancer across the seven major markets?
- What is the number of prevalent cases (2018-2034) of Triple-negative breast cancer by age across the seven major markets?
- What is the number of prevalent cases (2018-2034) of Triple-negative breast cancer by gender across the seven major markets?
- How many patients are diagnosed (2018-2034) with Triple-negative breast cancer across the seven major markets?
- What is the size of the Triple-negative breast cancer patient pool (2018-2023) across the seven major markets?
- What would be the forecasted patient pool (2024-2034) across the seven major markets?
- What are the key factors driving the epidemiological trend of Triple-negative breast cancer?
- What will be the growth rate of patients across the seven major markets?
Triple-negative Breast Cancer: Current Treatment Scenario, Marketed Drugs and Emerging Therapies
- What are the current marketed drugs and what are their market performance?
- What are the key pipeline drugs and how are they expected to perform in the coming years?
- How safe are the current marketed drugs and what are their efficacies?
- How safe are the late-stage pipeline drugs and what are their efficacies?
- What are the current treatment guidelines for Triple-negative breast cancer drugs across the seven major markets?
- Who are the key companies in the market and what are their market shares?
- What are the key mergers and acquisitions, licensing activities, collaborations, etc. related to the Triple-negative breast cancer market?
- What are the key regulatory events related to the Triple-negative breast cancer market?
- What is the structure of clinical trial landscape by status related to the Triple-negative breast cancer market?
- What is the structure of clinical trial landscape by phase related to the Triple-negative breast cancer market?
- What is the structure of clinical trial landscape by route of administration related to the Triple-negative breast cancer market?
This product will be updated with the latest data at the time of order. Consequently, dispatch time for this product will be 7-10 business days.
Table of Contents
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 129 |
| Published | May 2024 |
| Forecast Period | 2023 - 2034 |
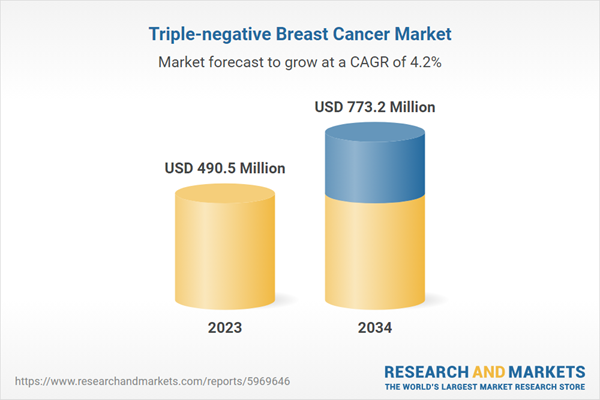
| Estimated Market Value ( USD | $ 490.5 Million |
| Forecasted Market Value ( USD | $ 773.2 Million |
| Compound Annual Growth Rate | 4.2% |
| Regions Covered | Global |