The epidermolysis bullosa market has been comprehensively analyzed in this report titled " Epidermolysis Bullosa Market: Epidemiology, Industry Trends, Share, Size, Growth, Opportunity, and Forecast 2024-2034". Epidermolysis bullosa (EB) refers to a rare genetic disorder that mainly affects the skin and mucous membrane. It is primarily caused by mutations in genes that produce proteins responsible for holding the layers of skin together. As a result, people suffering from the ailment have extremely fragile skin that blisters and tears up easily, even with minor trauma or friction. Depending on the affected layers of the skin and the severity, this condition is classified into EB simplex, junctional EB, dystrophic EB, and Kindler syndrome. Other common symptoms of the disease include thick or unformed nails, blisters inside the mouth and throat, hair loss, thin skin, tiny pimple-like bumps, dental problems, difficulty swallowing, itchy and painful skin, fused fingers, or toes, etc. The diagnosis of this disorder typically requires an evaluation of the patient's underlying indications and medical history. A skin biopsy and review of the tissue sample using advanced microscopic techniques are also recommended to help healthcare providers identify the layers of the skin affected and determine the type of EB.
The increasing prevalence of inherited diseases, which cause mutations in the genome and affect the strength and structure of connective tissues within the body, is primarily driving the epidermolysis bullosa market. In addition to this, the inflating demand for molecular genetic testing that can aid in early diagnosis of the disorder with more personalized patient care to minimize complexities is also bolstering the market growth. Furthermore, the widespread utilization of effective drugs, such as amitriptyline, gabapentin, morphine, etc., to treat severe types of EB and provide long-term pain relief is acting as another significant growth-inducing factor. Apart from this, the escalating application of genetically modified skin graft techniques, which enhance wound healing, restore skin functioning, and improve the appearance of damaged skin, is further creating a positive outlook for the market. Moreover, the rising popularity of protein replacement therapies for treating the condition, since they can replace the missing or defective protein to potentially reverse the structural and molecular defects in the skin, is expected to drive the epidermolysis bullosa market in the coming years.
This report provides an exhaustive analysis of the epidermolysis bullosa market in the United States, EU5 (Germany, Spain, Italy, France, and United Kingdom) and Japan. This includes treatment practices, in-market, and pipeline drugs, share of individual therapies, market performance across the seven major markets, market performance of key companies and their drugs, etc. The report also provides the current and future patient pool across the seven major markets. According to the report the United States has the largest patient pool for epidermolysis bullosa and also represents the largest market for its treatment. Furthermore, the current treatment practice/algorithm, market drivers, challenges, opportunities, reimbursement scenario and unmet medical needs, etc. have also been provided in the report. This report is a must-read for manufacturers, investors, business strategists, researchers, consultants, and all those who have any kind of stake or are planning to foray into the epidermolysis bullosa market in any manner.
Time Period of the Study
- Base Year: 2023
- Historical Period: 2018-2023
- Market Forecast: 2024-2034
Countries Covered
- United States
- Germany
- France
- United Kingdom
- Italy
- Spain
- Japan
Analysis Covered Across Each Country
- Historical, current, and future epidemiology scenario
- Historical, current, and future performance of the epidermolysis bullosa market
- Historical, current, and future performance of various therapeutic categories in the market
- Sales of various drugs across the epidermolysis bullosa market
Competitive Landscape:
This report also provides a detailed analysis of the current epidermolysis bullosa marketed drugs and late-stage pipeline drugs.In-Market Drugs
- Drug Overview
- Mechanism of Action
- Regulatory Status
- Clinical Trial Results
- Drug Uptake and Market Performance
Late-Stage Pipeline Drugs
- Drug Overview
- Mechanism of Action
- Regulatory Status
- Clinical Trial Results
- Drug Uptake and Market Performance
Key Questions Answered in this Report:
Market Insights
- How has the epidermolysis bullosa market performed so far and how will it perform in the coming years?
- What are the markets shares of various therapeutic segments in 2023 and how are they expected to perform till 2034?
- What was the country-wise size of the epidermolysis bullosa market across the seven major markets in 2023 and what will it look like in 2034?
- What is the growth rate of the epidermolysis bullosa market across the seven major markets and what will be the expected growth over the next ten years?
- What are the key unmet needs in the market?
Epidemiology Insights
- What is the number of prevalent cases (2018-2034) of epidermolysis bullosa across the seven major markets?
- What is the number of prevalent cases (2018-2034) of epidermolysis bullosa by age across the seven major markets?
- What is the number of prevalent cases (2018-2034) of epidermolysis bullosa by gender across the seven major markets?
- What is the number of prevalent cases (2018-2034) of epidermolysis bullosa by type across the seven major markets?
- How many patients are diagnosed (2018-2034) with epidermolysis bullosa across the seven major markets?
- What is the size of the epidermolysis bullosa patient pool (2018-2023) across the seven major markets?
- What would be the forecasted patient pool (2024-2034) across the seven major markets?
- What are the key factors driving the epidemiological trend of epidermolysis bullosa?
- What will be the growth rate of patients across the seven major markets?
Epidermolysis Bullosa: Current Treatment Scenario, Marketed Drugs and Emerging Therapies
- What are the current marketed drugs and what are their market performance?
- What are the key pipeline drugs and how are they expected to perform in the coming years?
- How safe are the current marketed drugs and what are their efficacies?
- How safe are the late-stage pipeline drugs and what are their efficacies?
- What are the current treatment guidelines for epidermolysis bullosa drugs across the seven major markets?
- Who are the key companies in the market and what are their market shares?
- What are the key mergers and acquisitions, licensing activities, collaborations, etc. related to the epidermolysis bullosa market?
- What are the key regulatory events related to the epidermolysis bullosa market?
- What is the structure of clinical trial landscape by status related to the epidermolysis bullosa market?
- What is the structure of clinical trial landscape by phase related to the epidermolysis bullosa market?
- What is the structure of clinical trial landscape by route of administration related to the epidermolysis bullosa market?
This product will be updated with the latest data at the time of order. Consequently, dispatch time for this product will be 7-10 business days.
Table of Contents
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 131 |
| Published | May 2024 |
| Forecast Period | 2023 - 2034 |
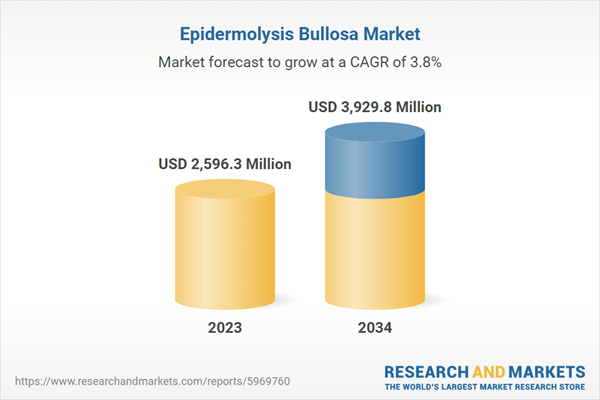
| Estimated Market Value ( USD | $ 2596.3 Million |
| Forecasted Market Value ( USD | $ 3929.8 Million |
| Compound Annual Growth Rate | 3.8% |
| Regions Covered | Global |